Minerva Neu (NASDAQ:NERV)
Minerva Neurosciences, Inc. (NERV), a clinical-stage biopharmaceutical company yesterday announced an amended agreement for MIN-202 used for Insomnia.
The agreement is with Janssen and states Minerva will gain global control for the development of MIN-202. It also states that Janssen will make cash payments up to $70 million, including $30 million upfront to Minerva.
Minerva Neurosciences, Inc. CEO’s Comments
“We view the new agreement with Janssen as a structure that will ensure a more focused and efficient clinical development of MIN-202 in insomnia by Minerva,” said Dr. Remy Luthringer, president and chief executive officer of Minerva. “We look forward to continuing our collaboration with Janssen while accelerating the clinical advancement of our portfolio. In addition, the infusion of financial resources under this agreement significantly extends Minerva’s financial runway.” Globe Newswire
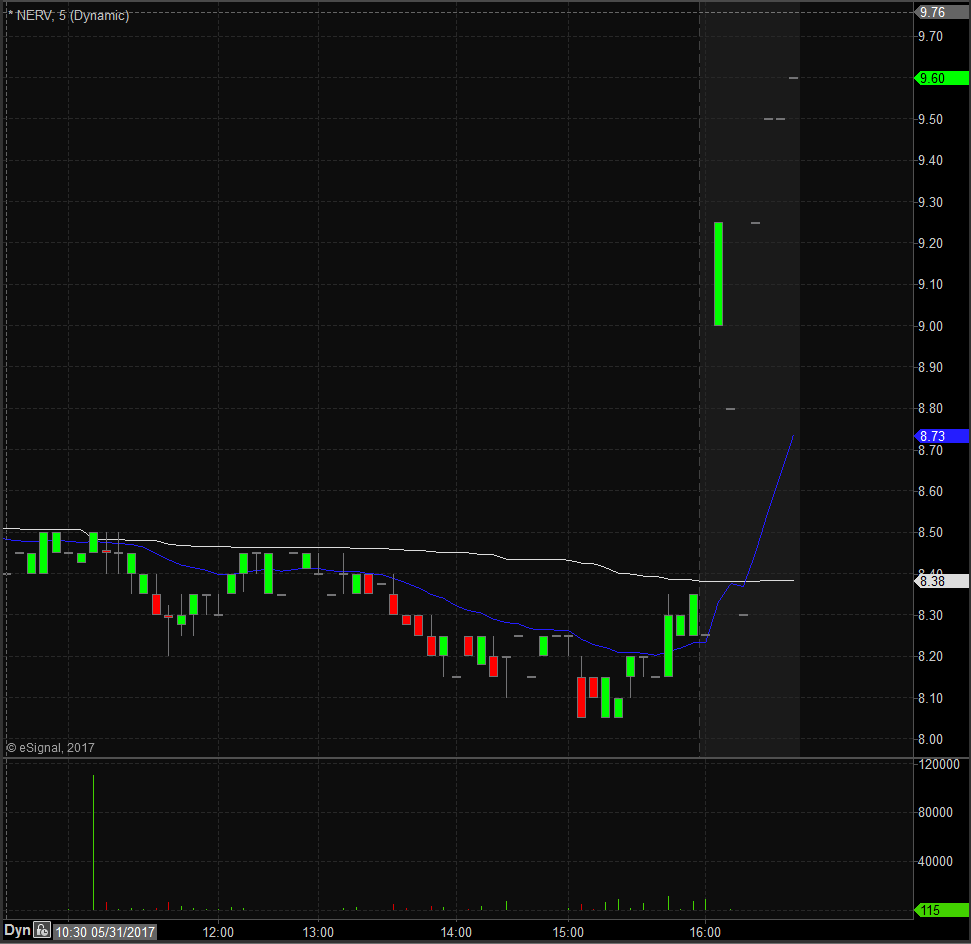
NERV Technical Analysis
NERV opened trading yesterday at $8.55 which was exactly the same as the previous day’s trading close. Shares closed trading yesterday at $8.25 and spiked up after market to $9.55, equivalent to a 16% increase from the closing price. Taking a look at the daily chart we can see the last time NERV traded above these levels we have to go back to February 17th when it traded at $9.90.
Taking a closer look at the daily chart we can see that before the spike up NERV had been in an overall downward trend dating back to November 25th, 2016 when it traded at $14.00. NERV has a float of 21.95 million shares and traded 1.75 times the normal daily trading volume on Wednesday.
For trading purposes, I would like to see NERV open trading on Thursday above $9.10 or the VWAP and if it does I would be looking to take a long position at the bell. My stop loss would be $0.20 from my entry position fearing anything more than that and the stock would start to fill in the gap up.
Company Profile
Minerva Neurosciences, Inc., a clinical-stage biopharmaceutical company, focuses on the development and commercialization of a portfolio of product candidates for the treatment of central nervous system diseases. The company’s lead product candidate includes MIN-101, a compound for the treatment of patients with schizophrenia that completed Phase IIb clinical trial.
It also offers MIN-202, which completed Phase IIa clinical trial for treating primary insomnia, as well as completed Phase 1b used for the treatment of major depressive disorder; and MIN-117, a compound that completed Phase IIa clinical trial for the treatment of patients suffering from major depressive disorder. The company’ preclinical stage product includes MIN-301, a soluble recombinant form of the Neuregulin-1b1 protein for the treatment of Parkinson’s disease.
Minerva Neurosciences, Inc. has a co-development and license agreement with Janssen Pharmaceutica, N.V. for the development of MIN-202. The company was formerly known as Cyrenaic Pharmaceuticals, Inc. and changed its name to Minerva Neurosciences, Inc. in 2013. Minerva Neurosciences, Inc. was founded in 2007 and is based in Waltham, Massachusetts.