Late stage springing forward
Mesoblast Ltd's (ASX:MSB) late-stage product pipeline is burgeoning. We expect a regulatory decision for its first product, MSC-100-IV in acute graft vs host disease (aGvHD) in Japan in mid-2015 following its filing last October, which would serve as critical confirmation of the company’s proprietary mesenchymal stem cell (MSC) technology. Two further products, MPC-150 in CHF and MPC-100-IV have entered pivotal Phase III trials over the past year. We increase our valuation of the company to A$3.41bn from A$3.16bn or A$10.51 per basic share from A$9.51 per basic share, representing significant upside to the current share price.
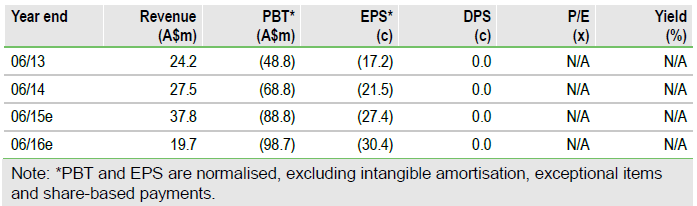
MSC-100-IV potentially first approved product
We expect a regulatory decision in mid-2015 in Japan for MSC-100-IV (JR-031) filed by Mesoblast’s partner JCR Pharmaceuticals for children and adults with aGvHD. Approval is expected on the basis of an expedited review process and would bring first commercial sales of the company’s adult stem cell therapies and milestones on royalties on sales.
Two pivotal Phase III trials ongoing
In January, Mesoblast announced the start of a Phase III trial for MPC-06 in low back pain. Additionally, Mesoblast’s partner Teva continues recruitment into a large Phase III study of MPC-150 in congestive heart failure, which commenced in 2014.
Solid financial footing
In the absence of new financing or partnerships, we estimate that Mesoblast’s cash holdings are sufficient to fund operations, including key late-stage trials, into late FY16. A faster uptake of MSC-100-IV in Japan (2015) and Canada (2016) than we currently forecast could extend its cash runway.
Valuation: rNPV of A$3.41bn (A$10.51/basic share)
Our estimated fair value share price of A$10.51 for Mesoblast, based on a risk-adjusted net present value (NPV) analysis, represents significant upside potential to the current price of A$3.65. Mesoblast’s share price has come down from its highs over the past year despite pipeline progression (two products have moved into pivotal Phase III trials). Upcoming share price inflection points include the regulatory decision for MSC-100-IV in aGvHD in Japan and the first interim analysis of the Phase III pivotal trials for MPC-150 in CHF.
To Read the Entire Report Please Click on the pdf File Below