Merck & Co., Inc. (NYSE:MRK) announced that the FDA has granted a priority review to yet another supplemental biologics license application (sBLA) for its PD-L1 inhibitor, Keytruda. With the latest sBLA filing, Merck is looking to get Keytruda approved for the first-line treatment of patients suffering recurrent or metastatic head and neck squamous cell carcinoma (HNSCC), both as a monotherapy or in combination with platinum and 5-fluorouracil (5-FU) chemotherapy.
With the FDA granting a priority review to the sBLA, a decision is expected on Jun 10, 2019. The approval will help Merck gain access to a broader patient population and boost sales for its blockbuster drug.
The sBLA was based on data from the phase III KEYNOTE-048 study wherein Keytruda as a monotherapy and also combined with chemotherapy demonstrated a significant improvement in overall survival (OS) as compared to the standard of care in the given patients, whose tumors expressed PD-L1 with CPS(combined proportion score)≥20 and CPS≥1.
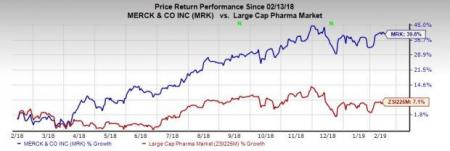
Shares of Merck have surged 39.8% in the past year, outperforming the industry’s increase of 7.1%.
Keytruda is already approved for many types of cancers and treatment settings including lung cancer, melanoma, head and neck cancer, classical Hodgkin’s lymphoma and bladder cancer.
In a very short span of time, Keytruda has become the largest product in Merck’s portfolio. The drug generated sales of $7.17 billion in 2018, reflecting a massive 88% surge year over year. Sales were driven by the launch of new indications globally. Keytruda sales are particularly gaining from a strong momentum in first-line lung cancer indication as it is the only anti-PD-1 medicine approved in first-line setting.
The Keytruda development program is also progressing well and the drug is being studied for more than 30 types of cancer in above 900 studies including 400 plus combination programs. Merck is collaborating with several companies, namely Amgen (NASDAQ:AMGN) , Incyte (NASDAQ:INCY) , Glaxo (NYSE:GSK) and Pfizer (NYSE:PFE), separately, for the evaluation of Keytruda in combination with other regimes.
Zacks Rank
Merck currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Zacks' Best Stock-Picking Strategy
It's hard to believe, even for us at Zacks. But from 2000-2018, while the market gained +4.8% per year, our top stock-picking strategy averaged +54.3% per year.
How has that screen done lately? From 2017-2018, it sextupled the market's +15.8% gain with a soaring +98.3% return.
Free – See the Stocks It Turned Up for Today >>
GlaxoSmithKline plc (GSK): Free Stock Analysis Report
Merck & Co., Inc. (MRK): Get Free Report
Amgen Inc. (AMGN): Free Stock Analysis Report
Incyte Corporation (INCY): Free Stock Analysis Report
Original post
Zacks Investment Research
