Merck (NYSE:MRK) and Japanese partner Easai Co., Ltd announced that the FDA has granted breakthrough therapy designation (“BTD”) to the combination regimen of Merck’s anti-PD-1 therapy, Keytruda, and Easai’s multiple receptor tyrosine kinase inhibitor, Lenvima.
The companies are developing the combination as a potential treatment for advanced and/or metastatic renal cell carcinoma (“RCC”) or kidney cancer.
This is the 12th BTD for Keytruda and second for Lenvima.
The designation aims to expedite the development and review of drugs intended to treat serious or life-threatening conditions and provide patients access to these as soon as possible.
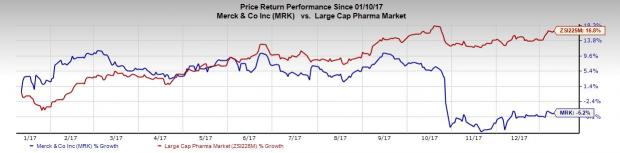
Merck’s shares have decreased 5.2% in the past year, underperforming the industry’s gain of 16.8% during that period.
The FDA granted the BTD to the combination regimen based on data from the RCC arm of the open label phase Ib/II study, Study 111, which is currently evaluating Keytruda+Lenvima in six cancer indications.
While Keytruda is Merck’s blockbuster immunotherapy drug approved for several cancer indications, Easai’s Lenvima is approved as a single agent for treating differentiated thyroid cancer and in combination with Novartis’ (NYSE:NVS) chemo drug, Afinitor (everolimus), for advanced RCC following one prior anti-angiogenic therapy.
Apart from Lenvima, Merck has collaborated with several companies including Amgen, Inc. (NASDAQ:AMGN) , Incyte, Glaxo and Pfizer, Inc. (NYSE:PFE) separately for the evaluation of Keytruda in combination with other regimens.
Merck carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Looking for Stocks with Skyrocketing Upside?
Zacks has just released a Special Report on the booming investment opportunities of legal marijuana.
Ignited by new referendums and legislation, this industry is expected to blast from an already robust $6.7 billion to $20.2 billion in 2021. Early investors stand to make a killing, but you have to be ready to act and know just where to look.
See the pot trades we're targeting>>
Pfizer, Inc. (PFE): Free Stock Analysis Report
Novartis AG (NVS): Free Stock Analysis Report
Merck & Company, Inc. (MRK): Free Stock Analysis Report
Amgen Inc. (AMGN): Free Stock Analysis Report
Original post