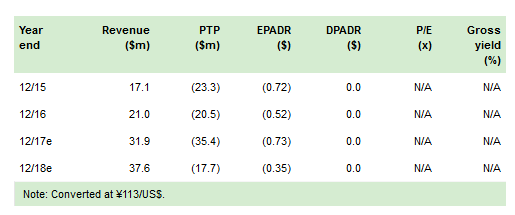
The license agreement between SymBio and The Medicines Company (NASDAQ:MDCO) for the exclusive rights to develop the IONSYS (SyB P-1501) pain patch in Japan has terminated effective 30 November. SymBio is seeking damages of at least $82m (¥9bn) arising from MDCO’s repudiation of the licence agreement. The termination is in line with our expectations after MDCO voluntarily withdrew IONSYS from sale in the US market in June; any compensation payments received from MDCO would represent upside to our forecasts and valuation. Our forecasts and valuation ($174m) are unchanged, as we have already removed all future costs and revenues for SyB P-1501 from our financial model.
Suspended SyB P-1501 Phase III to be terminated
SymBio in-licensed IONSYS (Syb P-1501) for the treatment of post-operative pain from MDCO in October 2015, and initiated a Phase III trial of SyB P-1501 in Japan in June 2016. SymBio suspended enrolment in the trial in April. In conjunction with the termination of the licence agreement, SymBio will terminate the development of SyB P-1501, a process that it expects to complete by 31 March 2018.
To read the entire report Please click on the pdf File Below: