On 14 June 2019, Oryzon Genomics SA (BS:ORYe) presented dose-finding data from the Phase II ALICE trial at the 24th Congress of the European Hematology Association (EHA-2019) in Amsterdam. The single-arm, open-label study enrolled newly diagnosed, elderly acute myeloid leukaemia (AML) patients and investigated iadademstat in combination with standard of care chemotherapy drug azacitidine. In addition to dose finding data, initial efficacy was also investigated. Overall, findings in this trial confirm the data seen in the first-in-man Phase I study Oryzon completed in late 2016 and support further investigation of an azacitidine/iadademstat combination in the second efficacy part of the ALICE trial.
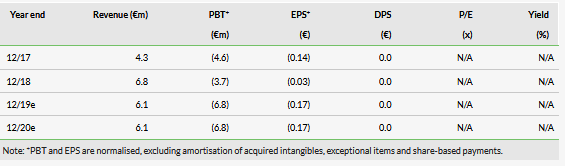
Dose-finding data set from Phase II ALICE trial
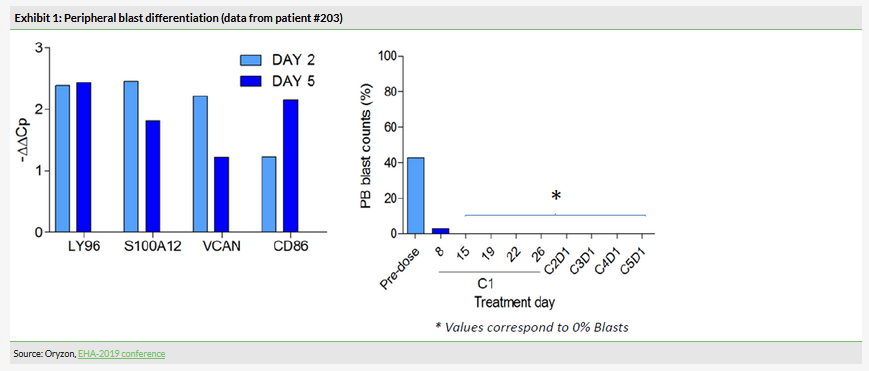
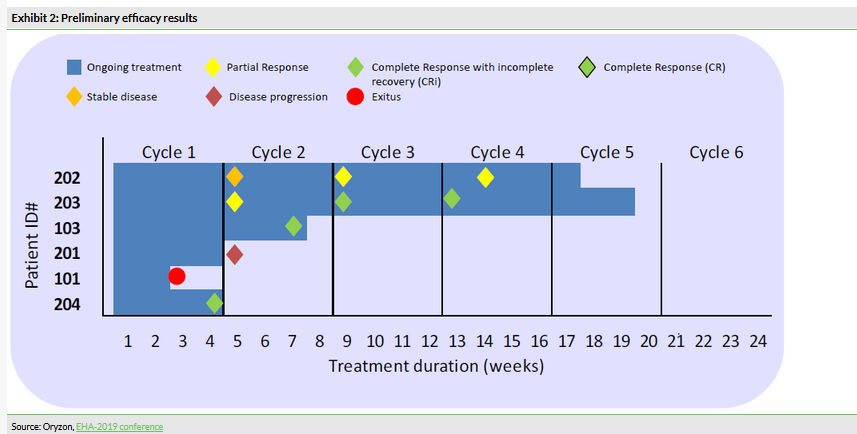
In this part of the study, which included six AML patients, the combination of iadademstat with azacitidine demonstrated a good safety profile and the recommended dose of 90 µg/m2 was established. This was the initially selected dose, therefore only six patients were needed. Iadademstat produced a clear differentiation effect in leukaemic blasts, ie turn them into normal blood cells (Exhibit 1). 80% of objective responses were observed in five evaluable patients (Exhibit 2). Of these, 75% (3/5) were complete remissions with incomplete haematologic recovery (CRi), while 25% (1/5) were partial remissions. Interestingly, the observed clinical responses appeared rapidly with a median time of 1.5 months.
Our take
Although the study was small and the focus was on establishing the recommended dose, the efficacy findings can be interpreted as showing potential. Notably, impaired differentiation of the leukaemic blasts is at the core of the pathophysiology of the disease. Iadademstat’s ability to induce the differentiation of blasts demonstrates it does what it was designed for. We note that reported overall response rates (ORR) in AML patients treated with azacitidine monotherapy are 25–32% depending on age (Seymour et al, 2016). A recently published article (DiNardo et al, 2019) described a clinical trial (n=145) where AML patients received venetoclax plus azacitidine or decitabine (both chemical analogs of cytidine) and the ORR was 67%. Venetoclax is a novel anticancer drug being developed by AbbVie/Genentech and the ORR of 67% compares well with the initial 80% rate observed in Oryzon’s trial. The second part of the ALICE trial should provide more insight in this regard.
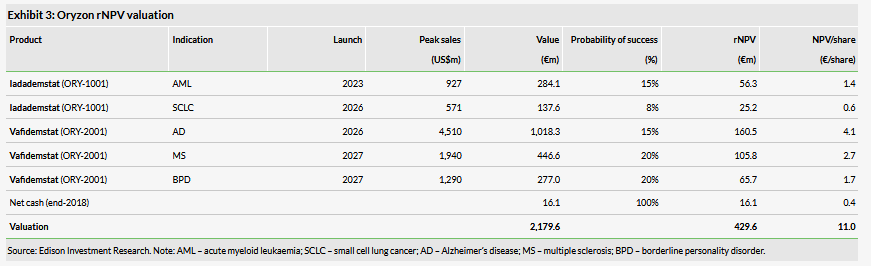
Valuation: €430m or €11.0/share
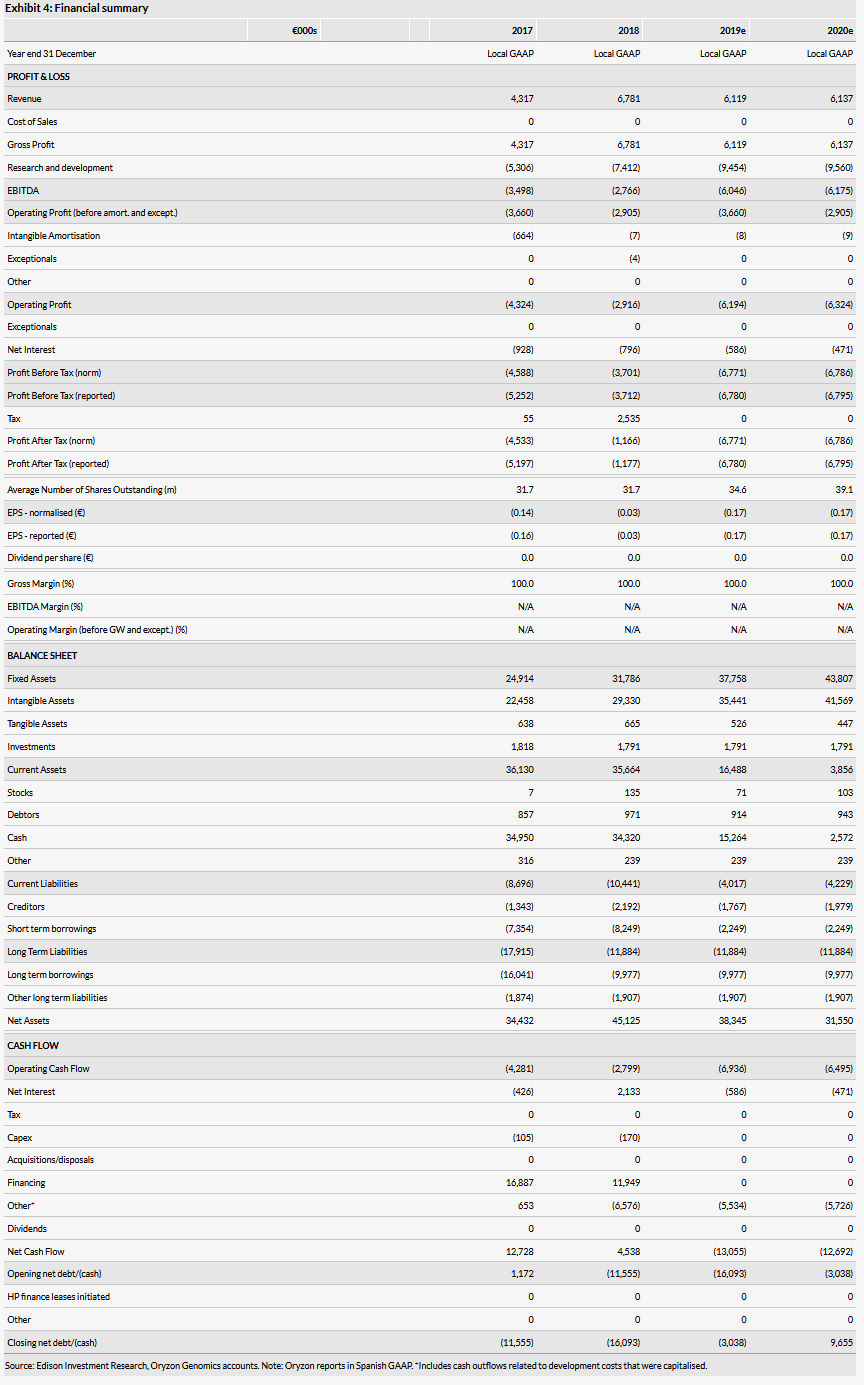
Our valuation remains €430m or €11.0/share and our forecasts are unchanged. The full results from the ALICE trial is the next catalyst in this indication and will prompt us to review our rNPV of the project including the success probability and other target populations as Oryzon indicated that one of the goals of the trial is to understand the broader application of iadademstat in other leukaemias.
Oryzon Genomics is a research client of Edison Investment Research Limited
Business description
Oryzon Genomics is a Spanish biotech focused on epigenetics. Iadademstat (Phase IIa) is being explored for acute leukaemias and SCLC; vafidemstat, its CNS product, is in Phase IIa trials in MS, AD and aggression. Newer asset ORY-3001 is being developed for certain orphan indications.
Next steps
The second part of the ALICE study will enrol up to 18 more patients and focus on the effectiveness of the drug combination. The next set of data should be presented at the ASH meeting in Orlando in December 2019. Iadademstat, as a selective LSD1 inhibitor, has been shown to be effective in preclinical models, including combinations with azacitidine. In addition, Oryzon has already completed a Phase I first-in-man trial, where iadademstat was given as a monotherapy, and demonstrated preliminary antileukaemic activity (reviewed in detail in our initiation report).