La Jolla Pharmaceutical (NASDAQ:LJPC)
La Jolla Pharmaceutical News
On Monday before the market opened, La Jolla Pharmaceutical announced positive phase 3 trial results from ATHOS-3 (Angiotensin II for the Treatment of High-Output Shock) for the treatment of hypotension with LJPC-501. This is great news for the company and the patients benefiting from this treatment. Shares are very active in the pre-market with well over 300k shares trading hands so far and over a 50% gain in value.
LJPC Technicals
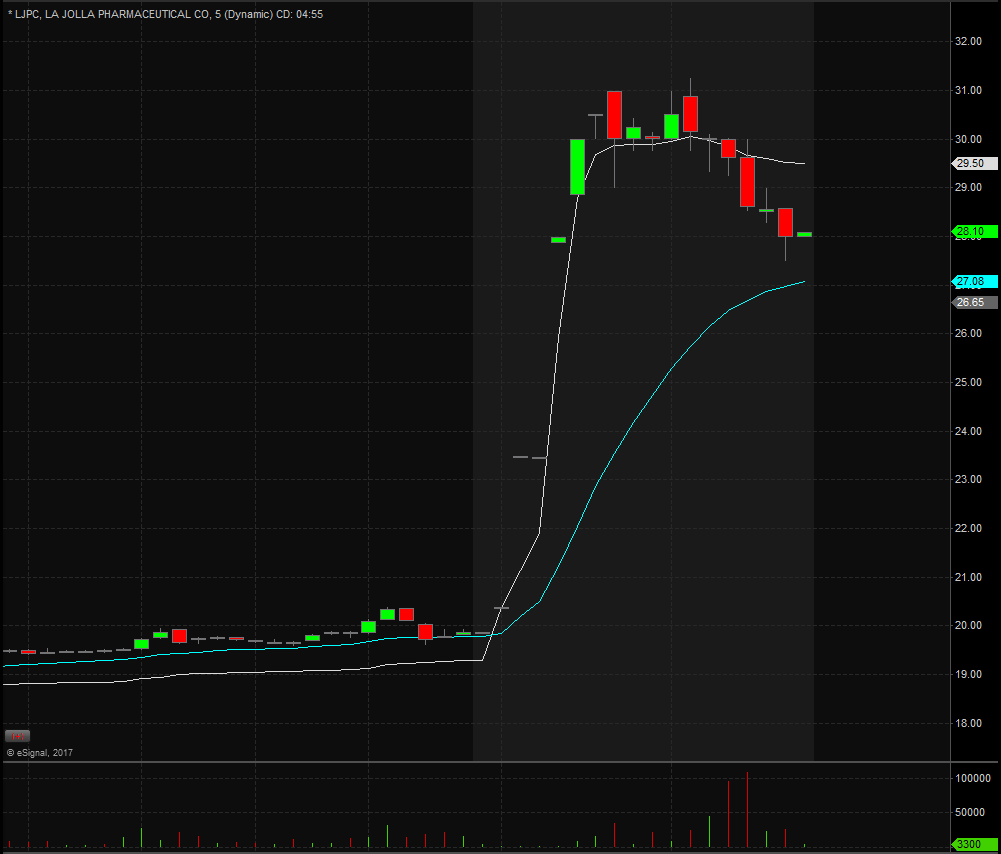
Shares of LJPC are surging in the pre-market with current highs at $31.25 after closing Friday at $19.87, marking a $57% jump in value over the weekend. Shares were up nearly 13% on heavy volume Friday indicating that someone knew about the news before hand. Shares haven’ traded at these levels since late in 2015.
Look for shares to be very volatile today as traders look to take advantage of the big movement. Generally after a big move like this in the pre-market, we tend to see a selloff at the open as longs look to take profits and shorts look to take contra positions against the extreme conditions.
A couple key levels to watch are the $28, $30, $36, $24 and $22. Keep this on your radar as it will be in play for Monday and most likely for the rest of the week as investors and traders fight to be on the right side of the move.
Comments On Phase 3 Results
“These study results support that angiotensin II, a molecule first synthesized by Dr. Irvine Page at the Cleveland Clinic, improves outcomes in distributive shock patients requiring high-dose catecholamines. Given the high mortality from this condition, it is important to offer physicians another potential treatment option,” said Daniel Sessler, M.D., the Michael Cudahy Professor and Chair of the Department of Outcomes Research at Cleveland Clinic.
“We are grateful to the patients, their families and the dedicated medical teams who contributed to this successful study,” said George F. Tidmarsh, M.D., Ph.D., president and chief executive officer of La Jolla. “We also are very appreciative of the FDA’s advice and contributions in the development of LJPC-501 and look forward to meeting with the FDA to discuss our NDA submission planned for the second half of this year.”
Company Profile
La Jolla Pharmaceutical Co. is a biopharmaceutical company, which engages in the discovery, development, and commercialization of innovative therapies intended to significantly improve outcomes in patients suffering from life-threatening diseases. Its products includes LJPC-501, LJPC-401, LJPC-30Sa and LJPC-30Sb. LJPC-501 is the proprietary formulation for angiotensin II. The LJPC-401 is the formulation of hepcidin, which is an endogenous peptide hormone. The LJPC-30Sa and LJPC-30Sb are the next generation gentamicin derivatives, which is an antibiotic for kidney toxicity. The company was founded in 1989 and is headquartered in San Diego, CA.
