Yesterday, big pharma player, Eli Lilly and Company (NYSE:LLY) reported better-than-expected second quarter results and raised its outlook for the year. However, shares were down 3% with investors focusing on the company’s update regarding its investigational rheumatoid arthritis (RA) treatment, baricitinib.
Here is a look at a few key takeaways from Lilly’s conference call.
How Did Key Drugs Perform?
Lilly’s second quarter 2017 revenues increased 8% from the year-ago quarter to $5.8 billion, topping the Zacks Consensus Estimate of $5.6 billion. New products like Trulicity, Taltz, Basaglar, Jardiance, Lartruvo and Cyramza performed well and drove volume growth. Late life cycle products like Cialis and Forteo drove price growth.
Alimta continued to face significant competitive headwinds while Zyprexa, Cymbalta, Evista, Strattera and Axiron were impacted by loss of exclusivity. Cialis was also affected by lower volume in the U.S. reflecting a decline in the overall erectile disorder (ED) market as well as the presence of generics.
Animal health revenue declined during the quarter reflecting market access pressure and competitive pressure in cattle and swine (Read more: Lilly Q2 Earnings Top, Baricitinib Re-Filing Delayed).
Baricitinib Facing Significant Delay
Lilly announced that there will be a delay of at least 18 months in the resubmission of the regulatory application for baricitinib, which is being developed in collaboration with Incyte (NASDAQ:INCY) . This means the resubmission will not take place this year.
The FDA had issued a complete response letter (CRL) for baricitinib earlier this year in April. At that time, the agency had told the companies that it is unable to approve baricitinib, a once-daily oral medication for the treatment of moderate-to-severe RA, in its current form. The FDA had asked for additional clinical data to determine the most appropriate doses as well as to further characterize safety concerns across treatment arms.
Although Lilly disagrees with the FDA's conclusions and believes the comprehensive clinical data supports a positive benefit-risk profile, the company will enter into discussions with the FDA to determine the kind of study needed to better define the benefit-risk profile of baricitinib.
Meanwhile, Lilly and Incyte have plans to explore baricitinib for other indications with a late-stage study in psoriatic arthritis slated to commence next year while studies in atopic dermatitis and lupus continue. Phase II atopic dermatitis data will be presented at a scientific forum by year end.
We note that baricitinib was approved in the EU under the trade name Olumiant in Feb 2017. The European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) recently agreed that the label should be updated to include a precaution for patients who have risk factors for deep venous thrombosis (DVT) and pulmonary embolism (PE).
Oncology R&D Strategy
Lilly provided an in-depth look at its oncology R&D strategy. The company’s strategy involves building on key treatments like Cyramza, Lartruvo and abemaciclib as foundational agents while it will focus on developing new standard-of-care changing therapies that clear a very high bar and will span a variety of mechanisms including immune-oncology. For now, Lilly will focus on seven candidates for priority internal development and three additional candidates (Ang2 MAb, CSF1R MAb and merestinib) with study data pending. The rest of the molecules in the pipeline will be partnered.
The seven molecules that have made it to Lilly’s priority development list include abemaciclib which is already under FDA review, TIM-3 MAb, PD-L1 MAb, Erk 1/2 inhibitor, TGFb RI KI, PI3/mTOR inhibitor and prexasertib. About 10 molecules are earmarked for external or partnered development -- while three of these assets are owned by third parties, Lilly is looking for partners for the remaining seven -- a FGFR-3 ADC, ralimetinib, a FGFR inhibitor, a notch inhibitor, galunisertib, a CXCR4 peptide antagonist and emibetuzumab.
Bottom Line
Although Lilly delivered a “beat and raise” quarter, the company’s results got overshadowed by the baricitinib update. Investors were disappointed by the delay in the resubmission of the baricitinib regulatory filing in the U.S. with the company saying that there could be a minimum delay of 18 months. With several products facing generic competition and competitive pressure increasing, Lilly needs to focus on bringing new products to market. The company’s decision to prioritize its oncology pipeline makes sense. Lilly is a Zacks Rank #2 (Buy) stock -- you can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here. Year-to-date, Lilly’s shares have gained 11.8%, just about topping the industry’s 11.2% rally.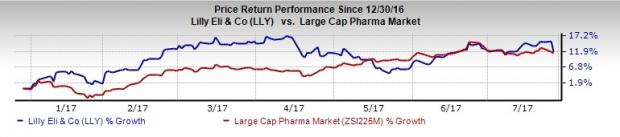
Watch out for earnings reports from other large cap pharma companies like Merck & Co., Inc. (NYSE:MRK) and Pfizer Inc. (NYSE:PFE) . Merck, a Zacks Rank #2 stock has a pretty good earnings track record having surpassed expectations in each of the last four quarters with an average surprise of 4.36%. Merck will be reporting Q2 results on Jul 28.
Pfizer’s track record is mixed with the company topping expectations in two of the last four quarters. Pfizer, which will be reporting on Aug 1, has a positive Earnings ESP of 1.54% for the second quarter.
The Hottest Tech Mega-Trend of All
Last year, it generated $8 billion in global revenues. By 2020, it's predicted to blast through the roof to $47 billion. Famed investor Mark Cuban says it will produce "the world's first trillionaires," but that should still leave plenty of money for regular investors who make the right trades early.
See Zacks' 3 Best Stocks to Play This Trend >>
Pfizer, Inc. (PFE): Free Stock Analysis Report
Eli Lilly and Company (LLY): Free Stock Analysis Report
Merck & Company, Inc. (MRK): Free Stock Analysis Report
Incyte Corporation (INCY): Free Stock Analysis Report
Original post
Zacks Investment Research
