Intercept Pharmaceuticals, Inc. (NASDAQ:ICPT) reported a loss of $3.46 per share in the second quarter of 2017, narrower than the Zacks Consensus Estimate of a loss of $3.62 but wider than the year-ago loss of $3.14.
Quarterly revenues were $30.9 million, up significantly from $5.5 million in the year-ago quarter and beat the Zacks Consensus Estimate of $27.0 million.
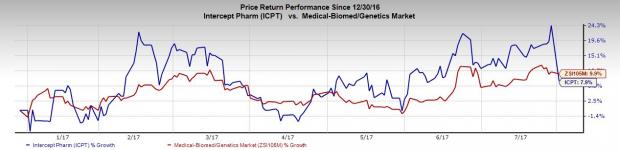
Intercept’s share price movement in the year so far indicates that the stock has underperformed the industry. The company’s shares have gained 7.9% during this period, lower than the industry’s gain of 9.9%.
Quarter in Detail
Ocaliva recorded $30.4 million of sales in the second quarter of 2017, up from$20.6 million sales in the first quarter of 2017. Note that in May 2016, Ocaliva was approved in the U.S., in combination with ursodeoxycholic (UDCA) for the treatment of primary biliary cholangitis (PBC) in adults with an inadequate response to UDCA, or as monotherapy in adults who are unable to endure UDCA. The drug was also granted conditional approval by the European Commission in Dec 2016.
Sales from the U.S. markets came in at $27.9 million, up from $19.8 million recorded in the first quarter as prescriptions continue to grow steadily. Revenues from international markets were $2.5 million driven mainly by sales in Germany and France.
Research and development expenses increased 26.6% year over year to $44.2 million primarily driven by increases in clinical development programs for OCA and infrastructure to support such programs.
Selling, general and administrative expenses increased to $66.9 million, up from $48.7 million in the year-ago quarter driven by expenses related to Ocaliva’s commercialization activities and additional personnel-related costs to support commercial and international initiatives.
2017 Outlook Reiterated
Intercept expects operating expenses to be in the range of $380–$420 million in 2017.The company believes that continued commercialization of Ocaliva in PBC in the United States and other markets, sustained clinical development for OCA in PBC and NASH and the continued advancement of INT-767 and other pipeline programs are likely to drive growth.
Demand in the third quarter will however reflect the typical summer trends but is expected to pick up again in the fourth quarter.
Pipeline Update
Ocaliva is being evaluated for other indications including non-alcoholic steatohepatitis (NASH) and primary sclerosing cholangitis (PSC).
Concurrent with the second-quarter earnings, Intercept announced trial results from two phase II trials–CONTROL (Combination OCA aNdsTatins for monitoRing Of Lipids) and AESOP for PSC. CONTROL study is being conducted to evaluate the effect of Ocaliva in combination with statin therapy on lipid metabolism in patients with NASH. Results from AESOP revealed that OCA met the primary endpoint of statistically significant reduction in alkaline phosphatase (ALP) while results from CONTROL showed that the company achieved its objective in demonstrating that the lowest available dose of atorvastatin rapidly reverses OCA associated LDL changes to below baseline levels in nonalcoholic steatohepatitis (NASH) patients with fibrosis or cirrhosis.
The FDA has earlier approved a redesign of the phase III trial, REGENERATE on Ocaliva for the safety and efficacy in treating NASH patients with liver fibrosis. The company now needs to achieve only one co primary endpoint- either fibrosis improvement or NASH resolution as compared withthe earlier target of achieving both.The sample size of the trial has also been reduced to approximately 750 patients or about 250 patients per arm. The company completed enrolment for the interim analysis cohort in the REGENERATE trial (data readout in the first half of 2019).
The company also plans to initiate a phase III trial on Ocaliva in NASH patients with cirrhosis in the second half of 2017. The company will also initiate a phase II trial on another candidate, INT-767, in NASH patients with fibrosis in the second half of 2017.The company also continues to enrol in its phase IV trial, COBALT, to confirm Ocaliva's clinical benefit on outcomes in PBC.
Our Take
Intercept’s second-quarter results were encouraging as the company reporteda narrower-than-expected loss and beat on revenues. Demand for Ocalivais steadily increasing and we expect sales of the drug should pick up further in 2017 as international demand picks up in the second half. However, expenses are expected to rise as the company plans to invest in commercial activities related to Ocaliva.
We remind investors that some other companies like Novartis AG (NYSE:NVS) and Gilead Sciences, Inc. (NASDAQ:GILD) have FXR agonists in phase II or earlier stages of clinical or preclinical development that canbe used to treat PBC, NASH and the other liver diseases.
Zacks Rank & Key Pick
Intercept currently carries a Zacks Rank #3 (Hold).
A better-ranked stock in the healthcare sector is Exelixis, Inc. (NASDAQ:EXEL) which currently carries a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Exelixis’s earnings per share estimates inched up from 53 cents to 55 cents for 2018, over the last 30 days. The company has delivered positive earnings surprises in all the trailing four quarters with an average beat of 512.11%.
Will You Make a Fortune on the Shift to Electric Cars?
Here's another stock idea to consider. Much like petroleum 150 years ago, lithium power may soon shake the world, creating millionaires and reshaping geo-politics. Soon electric vehicles (EVs) may be cheaper than gas guzzlers. Some are already reaching 265 miles on a single charge.
With battery prices plummeting and charging stations set to multiply, one company stands out as the #1 stock to buy according to Zacks research.
It's not the one you think.
Novartis AG (NVS): Free Stock Analysis Report
Gilead Sciences, Inc. (GILD): Free Stock Analysis Report
Exelixis, Inc. (EXEL): Free Stock Analysis Report
Intercept Pharmaceuticals, Inc. (ICPT): Free Stock Analysis Report
Original post
Zacks Investment Research