On Jan 25, we issued an updated research report on Inovio Pharmaceuticals, Inc. (NASDAQ:INO) .
Inovio’s most advanced candidate, VGX-3100 vaccine, has been progressing well alongside other promising pipeline candidates in its portfolio.
VGX-3100 is currently being evaluated in a phase III study (REVEAL 1) for the treatment of cervical dysplasia, caused by human papillomavirus (HPV). The company expects to complete enrollment in REVEAL 1 study by early 2019 and initiate the REVEAL 2 study thereafter. Another phase II study is examining the efficacy of VGX-3100 on women with vulvar dysplasia and anal dysplasia. This will be the third indication that VGX-3100 will be evaluated for.
Last August, Inovio announced a partnership with the AIDS Malignancy Consortium to evaluate VGX-3100 for treating HPV-associated precancerous conditions in patients, who have tested positive for HIV.
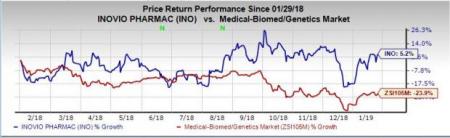
Shares of Inovio have increased 5.2% in the past year against the industry’s decrease of 23.9%.
Inovio out-licensed MEDI0457 to MedImmune, a subsidiary of AstraZeneca plc (NYSE:AZN) . MEDI0457 is a combination of VGX-3100 and its DNA-based IL-12 cytokine.
Inovio is set to receive an undisclosed milestone payment from AstraZeneca as the latter dosed the first patient in a phase II study on T cell-activating immunotherapy, MEDI0457. The program will evaluate MEDI0457 in combination with AstraZeneca’s anti PD-L1 checkpoint inhibitor, Imfinzi, for treating several HPV related cancers.
Apart from VGX-3100, Inovio has several vaccine candidates in its pipeline under early-to-mid-stage development.
Earlier this month, Inovio initiated the first human study of its DNA-encoded monoclonal antibody (dMAb) technology to prevent Zika virus infection in collaboration with The Wistar Institute and the University of Pennsylvania. The phase I dose-escalation study on INO-A002 demonstrated safety and tolerability, starting at lower level and then gradually increasing the doses.
Last week, Inovio announced that a second patient with HPV-related head and neck cancer treated with INO-3112 in a phase I analysis achieved a sustained complete response after subsequent treatment with a PD-1 checkpoint inhibitor. The complete responder received Merck's (NYSE:MRK) Keytruda, a PD-1 inhibitor, after being administered with INO-3112. The first complete responder received Bristol-Myers Squibb's (NYSE:BMY) Opdivo after the vaccine.
Inovio is developing a vaccine for Ebola virus (INO-4212). The vaccine provided 100% protection following a lethal dose in a preclinical study and prompted long-term immune responses in monkeys.
Inovio’s INO-4700 vaccine is being co-developed by GeneOne Life Science Inc for MERS (Middle East Respiratory Syndrome). The company expects to begin a phase II probe on MERS vaccine in the Middle East during 2019. Inovio and GeneOne Life Science also dosed the first patient in a phase I investigation on its hepatitis C vaccine, GLS-6150, to boost immunity in patients.
Inovio is evaluating its HIV vaccine, Pennvax-GP’s, in a phase I/II study and dosed the first patient with the same. The vaccine targets all major HIV strains with potential capacity to improve the human immune system and control HIV without antiretroviral therapy.
Inovio has collaboration with a few big pharma companies, which help it with constant funding for pipeline development. However, Inovio has no approved product in its portfolio. Hence, an excessive dependence on its partners for developing its pipeline candidates remains an overhang on the company.
Zacks Rank
Inovio currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Zacks' Top 10 Stocks for 2019
In addition to the stocks discussed above, would you like to know about our 10 finest buy-and-holds for the year?
Who wouldn't? Our annual Top 10s have beaten the market with amazing regularity. In 2018, while the market dropped -5.2%, the portfolio scored well into double-digits overall with individual stocks rising as high as +61.5%. And from 2012-2017, while the market boomed +126.3, Zacks' Top 10s reached an even more sensational +181.9%.
AstraZeneca PLC (AZN): Free Stock Analysis Report
Merck & Co., Inc. (MRK): Free Stock Analysis Report
Bristol-Myers Squibb Company (BMY): Get Free Report
Inovio Pharmaceuticals, Inc. (INO): Get Free Report
Original post
Zacks Investment Research
