Incyte Corporation (NASDAQ:INCY) announced that the FDAaccepted and granted Priority Review to the new drug application (NDA) for capmatinib, which is an investigational, oral selective MET inhibitor. The company is seeking approval of capmatinib for the treatment of first-line and previously treated patients with locally advanced or metastatic MET exon 14 skipping (METex14) mutated non-small cell lung cancer (NSCLC) — a type of lung cancer with a particularly poor prognosis. If approved, the candidate will be the first therapy to specifically target METex14 mutated advanced lung cancer.
The Priority Review designation from the FDA is generally granted to drugs that have the potential to provide significant improvements in the safety and effectiveness of the treatment, prevention or diagnosis of a serious disease. This designation shortens the FDA review period following the acceptance of the NDA to six months compared to 10 months for standard review.
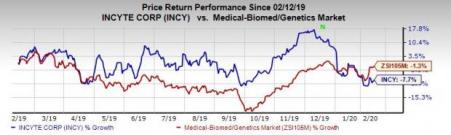
Shares of the company have lost 7.7% in the past year compared with the industry’s decline of 1.3%.
The NDA submission was supported by positive primary results from the GEOMETRY mono-1 study.. Data from the study showed that treatment with capmatinib demonstrated promising efficacy in patients with locally advanced or metastatic NSCLC that harbors MET exon-14 skipping mutation.
The primary endpoint was overall response rate (ORR)based on the Blinded Independent Review Committee (BIRC) assessment per RECIST v1.1. The overall response rate among treatment-naive patients receiving capmatinib was 68% and 41% for previously treated patients. The median duration of response was also clinically meaningful, irrespective of the prior line of therapy.
In September 2019, the FDA also granted Breakthrough Therapy designation to capmatinib for the first-line treatment of patients with metastatic MET exon14 skipping-mutated NSCLC.
We remind investors that capmatinib was licensed to Novartis (NYSE:NVS) by Incyte in 2009. Per the agreement, Incyte granted Novartis exclusive worldwide development and commercialization rights to capmatinib and certain back-up compounds in all indications.
Also, competition is stiff in the NSCLC market with several big pharma companies having approved drugs for the same, including Merck’s (NYSE:MRK) Keytruda, AstraZeneca’s (NYSE:AZN) Tagrisso and Roche’s Tecentriq.
Incyte currently has a Zacks Rank #4 (Sell). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Zacks Top 10 Stocks for 2020
In addition to the stocks discussed above, would you like to know about our 10 finest buy-and-hold tickers for the entirety of 2020? Last year's 2019 Zacks Top 10 Stocks portfolio returned gains as high as +102.7%. Now a brand-new portfolio has been handpicked from over 4,000 companies covered by the Zacks Rank. Don’t miss your chance to get in on these long-term buys.
Access Zacks Top 10 Stocks for 2020 today >>
AstraZeneca PLC (AZN): Free Stock Analysis Report
Merck & Co., Inc. (MRK): Free Stock Analysis Report
Incyte Corporation (INCY): Free Stock Analysis Report
Novartis AG (NVS): Free Stock Analysis Report
Original post
Zacks Investment Research