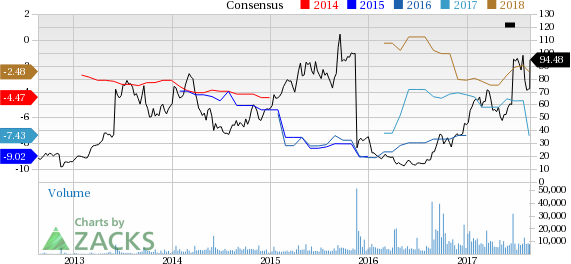
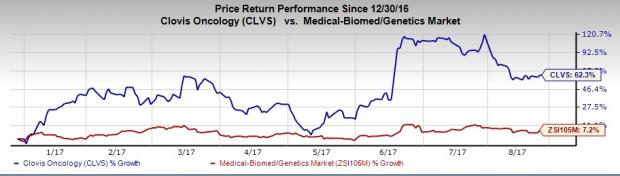
Shares of Clovis Oncology, Inc. (NASDAQ:CLVS) have witnessed a continuous upside this year. Shares of this small biotech company are up 62.3% so far this year, outperforming the 7.2% increase registered by the industry during this period. Let’s analyze the factors that have driven the rally so far.
The company’s only approved drug, Rubraca, has shown an impressive growth trend in 2017. The drug received accelerated approval in Dec 2016. The company’s commercialization efforts have helped the ovarian cancer drug register almost 100% growth in sales sequentially in the second quarter of 2017. Rubraca sales were $21.7 million in the first half of 2017. The company had 1100 new patients on therapy in the period.
Rubraca is a PARP inhibitor, which is approved as a monotherapy for the treatment of advanced ovarian cancer in patients who have been treated with two or more chemotherapies. The patients are selected for therapy based on an FDA-approved companion diagnostic for Rubraca. The drug was in-licensed from Pfizer Inc. (NYSE:PFE) in 2011.
Two confirmatory studies - ARIEL3 and ARIEL4 – are being conducted by Clovis for converting the accelerated approval to continued approval of Rubraca.
The company’s shares got a boost when it announced positive top-line results from ARIEL 3 in June 2017. Promising progression-free survival (PFS) and safety results from the pivotal maintenance confirmatory study demonstrated that Rubraca had a meaningful impact in delaying disease recurrence in advanced ovarian cancer patients.
Clovis is planning to file a supplemental new drug application (sNDA) to the FDA by October this year based on ARIEL-3 data to include second-line or later maintenance indication for advanced ovarian cancer on the label of Rubraca. The company expects the label expansion to increase patient population by at least four times.
Meanwhile, the other phase III confirmatory study -- ARIEL4 -- is evaluating Rubraca versus chemotherapy in patients who have failed two prior lines of therapy. Rubraca is also under review in the EU for a comparable ovarian cancer indication. An approval is expected in EU in the first quarter of 2018 and Clovis is establishing the commercial infrastructure for the same.
There is immense commercial potential for Rubraca in the target market due to increasing demand for PARP inhibitors. Per the American Cancer Society, ovarian cancer ranks fifth in deaths from cancer among women. Over 22,440 cases of ovarian cancer are estimated to be diagnosed in the U.S. in 2017. There is a huge unmet need for new treatment options, given that one in four women with ovarian cancer has a germline or somatic BRCA mutation.
Rubraca is also being developed in additional cancer indications either as monotherapy or in combination with other agents, including Tecentriq-Rubraca combination in gynecologic cancers. This combination study is sponsored by Roche. Moreover, in Jul 2017 the company collaborated with Bristol-Myers Squibb Company (NYSE:BMY) to evaluate Rubraca in combination with the latter’s Opdivo in ovarian, breast and prostate cancer.
It is important to note that the ovarian cancer market is already crowded with the presence of major players. With the launch of Tesaro, Inc.’s (NASDAQ:TSRO) Zejula in Apr 2017, competition has intensified further.
Clovis currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
4 Surprising Tech Stocks to Keep an Eye On
Tech stocks have been a major force behind the market’s record highs, but picking the best ones to buy can be tough. There’s a simple way to invest in the success of the entire sector. Zacks has just released a Special Report revealing one thing tech companies literally cannot function without.
More importantly, it reveals 4 top stocks set to skyrocket on increasing demand for these devices. I encourage you to get the report now – before the next wave of innovations really takes off.
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Pfizer, Inc. (PFE): Free Stock Analysis Report
Clovis Oncology, Inc. (CLVS): Free Stock Analysis Report
TESARO, Inc. (TSRO): Free Stock Analysis Report
Original post
Zacks Investment Research