Roche Holdings AG (OTC:RHHBY) announced that the FDA has approved Actemra (tocilizumab) subcutaneous injection for the treatment of giant cell arteritis (GCA).
As per Roche, Actemra is the first therapy approved by the FDA for the treatment of adult patients with GCA.
The approval for GCA is the sixth indication for which Actemra obtained FDA approval. The approval was based on positive results from the phase III study, GiACTA.
As per estimates, GCA affects nearly 228,000 people who are more than 50 years old in the U.S.
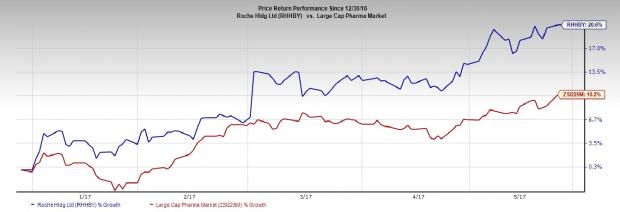
Roche’s share price shows that the company has outperformed the Zacks classified industry year to date. The stock is up 20.6% compared with the Large Cap Pharmaceuticals industry’s gain of 10.2%.
We remind investors that Actemra is already approved for the treatment of adult patients with moderately to severely active rheumatoid arthritis (RA) who have used one or more disease-modifying antirheumatic drugs (DMARDs), such as methotrexate (MTX) that did not provide enough relief. Actemra is also used as an IV formulation for patients with active polyarticular juvenile idiopathic arthritis (PJIA) or systemic juvenile idiopathic arthritis (SJIA) two years of age or older.
Moreover, Actemra/RoActemra is also being studied in a global phase III multicentre, randomised, double-blind, placebo-controlled study (NCT02453256) for patients with systemic sclerosis (SSc) also known as scleroderma. The drug was granted Breakthrough Therapy Designation for SSc by the FDA in Jun 2015.
Roche has a strong presence in the oncology market. The company dominates the breast cancer space with strong demand for HER2 franchise drugs with drugs like Herceptin, Perjeta and Kadcyla.
We are also impressed by the company's efforts to develop its portfolio beyond oncology into immunology. Its immunology portfolio currently comprises drugs like MabThera/Rituxan and Actemra for RA, Xolair for asthma, Pulmozyme for cystic fibrosis and Esbriet for idiopathic pulmonary fibrosis (IPF), along with a promising pipeline that includes etrolizumab for inflammatory bowel disease, and lebrikizumab for severe asthma, atopic dermatitis and IPF. Actemra has proven to be a strong growth driver for the company, while Xolair continues to gain market share.
Zacks Rank & Key Picks
Roche currently carries a Zacks Rank #3 (Hold). Some better-ranked stocks in the health care sector include VIVUS, Inc. (NASDAQ:VVUS) , sporting a Zacks Rank #1 (Strong Buy),and Aldeyra Therapeutics, Inc. (NASDAQ:ALDX) and Zoetis Inc. (NYSE:ZTS) ,carrying Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks Rank #1 stocks here.
VIVUS’ loss per share estimates narrowed from 50 cents to 39 cents for 2017 over the last 60 days. The company posted positive earnings surprises in all of the four trailing quarters, with an average beat of 233.69%.
Aldeyra’ loss per share estimates narrowed from $1.79 to $1.69 cents for 2017 over the last 60 days. The company posted positive earnings surprises in three of the four trailing quarters, with an average beat of 7.8%.
Zoetis’ earnings per share estimates increased from $2.32 to $2.34 for 2017 over the last 60 days. The company posted positive earnings surprises in all of the four trailing quarters, with an average beat of 9.82%.
Zacks' 2017 IPO Watch List
Before looking into the stocks mentioned above, you may want to get a head start on potential tech IPOs that are popping up on Zacks' radar. Imagine being in the first wave of investors to jump on a company with almost unlimited growth potential? This Special Report gives you the current scoop on 5 that may go public at any time.
One has driven from 0 to a $68 billion valuation in 8 years. Four others are a little less obvious but already show jaw-dropping growth. Download this IPO Watch List today for free >>
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
VIVUS, Inc. (VVUS): Free Stock Analysis Report
Zoetis Inc. (ZTS): Free Stock Analysis Report
Aldeyra Therapeutics, Inc. (ALDX): Free Stock Analysis Report
Original post
Zacks Investment Research
