Gilead Sciences, Inc. (NASDAQ:GILD) presented a series of data at the 2019 Conference on Retroviruses and Opportunistic Infections (CROI) in Seattle.
The company announced results from two studies evaluating the resistance profile of a once-daily single tablet HIV regimen, Biktarvy (bictegravir 50 mg/emtricitabine 200 mg/tenofovir alafenamide 25 mg tablets, BIC/FTC/TAF) in virologically suppressed adults switching from dolutegravir/abacavir/lamivudine (DTG/ABC/3TC) or a boosted protease inhibitor (PI)-based regimen for the treatment of HIV-1.
The results showed high rates of virologic suppression with Biktarvy in treatment-experienced adults, regardless of pre-existing resistance to nucleoside reverse transcriptase inhibitors (NRTIs).
We note that Biktarvy is already approved in the United States as a complete regimen for the treatment of HIV-1 infection in adults without any antiretroviral treatment history. Biktarvy is also approved to replace the current antiretroviral regimen in those adults who are virologically suppressed on a stable antiretroviral regimen for at least three months.
Gilead announced 48-week results from a phase II/III study evaluating the efficacy and safety of Biktarvy in virologically suppressed adolescents and children at least 6 years of age infected with HIV. Biktarvy maintained high rates of virologic suppression with a low incidence of study drug-related adverse events and no treatment-emergent resistance at week 48.
Gilead also announced results from the phase III randomized, controlled, double-blind study evaluating the safety and efficacy of the investigational use of once-daily Descovy for HIV pre-exposure prophylaxis (PrEP) compared with Truvada, in men who have sex with men and transgender women at risk of sexually acquired HIV infection. Descovy met the pre-established criteria for non-inferiority to Truvada using a stringent rate ratio statistical comparison.
Moreover, results showed that participants receiving Descovy have significant advantages with respect to bone and renal laboratory parameters, which were pre-specified secondary endpoints, compared with those receiving Truvada. Descovy is already approved in combination with other antiretroviral agents for the treatment of HIV infection in patients weighing 35 kg or more but is not indicated for PrEP. Truvada is indicated in combination with safer sex practices for HIV PrEP to reduce the risk of sexually acquired HIV in at-risk adults and adolescents weighing 35 kg or more.
Gilead plans to file regulatory applications for Descovy for the PrEP indication as a potential important new option to prevent individuals from getting infected and contribute to the achievement of national and global HIV prevention goals.
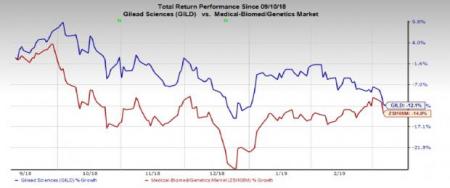
Gilead’s stock has lost 12.1% in the past six months compared with the industry's decline of 14%.
Gilead’s HIV franchise maintains momentum, driven by the rapid adoption of Descovy-based regimens and Biktarvy. However, Gilead will have to generate substantial revenues from its HIV franchise to offset the HCV sales decline. This will be a challenging task for the company with stiff competition from the likes of GlaxoSmithKline (NYSE:GSK) in the HIV market.
Given the persistent decline in HCV sales, the company is looking to HIV and newer avenues to help the top line. The initial uptake of Yescarta (from the Kite Pharma acquisition) is also encouraging but it will take some time for sales to contribute significantly to the top line, given the high cost of the treatment. Moreover, Novartis’ (NYSE:NVS) Kymriah is also there in the market, posing competition.
Gilead also collaborated with Agneus (NASDAQ:AGEN) for the development and commercialization of up to five novel immuno-oncology therapies.
Zacks Rank
Gilead currently carries a Zacks Rank #4 (Sell). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Is Your Investment Advisor Fumbling Your Financial Future?
See how you can more effectively safeguard your retirement with a new Special Report, “4 Warning Signs Your Investment Advisor Might Be Sabotaging Your Financial Future.”
GlaxoSmithKline plc (GSK): Free Stock Analysis Report
Novartis AG (NVS): Free Stock Analysis Report
Gilead Sciences, Inc. (GILD): Free Stock Analysis Report
Agenus Inc. (AGEN): Free Stock Analysis Report
Original post
Zacks Investment Research
