Gilead Sciences, Inc. (NASDAQ:GILD) announced that the FDA has granted priority review for the company’s New Drug Application (NDA) for an investigational, fixed-dose combination of bictegravir (50mg) (BIC), a novel investigational integrase strand transfer inhibitor (INSTI), and emtricitabine/tenofovir alafenamide (200/25mg) (FTC/TAF), a dual-NRTI backbone, for the treatment of HIV-1 infection.
The NDA was filed on Jun 12, 2017. The FDA has set a target action date under the Prescription Drug User Fee Act (PDUFA) of Feb 12, 2018.
A marketing application for BIC/FTC/TAF is also under review in the European Union, and was validated by the European Medicines Agency (EMA) in July.
A potential approval is expected toboost Gilead’s HIV portfoliofurther.
Gilead is a dominant player in the HIV market with an impressive portfolio for the same.
The newly launched TAF-based products Genvoya, Odefsey and Descovy are performing well with strong adoption in both the U.S. and EU. Genvoya has already become the most-prescribed regimen for both treatment-naïve and switch patients since its launch in Nov 2015. The TAF-based regimens now represent 51% of total Gilead HIV prescription volume following the launch of Genvoya and Odefsey and Descovy in 2016. Genvoya is now the company’s bestselling HIV product, surpassing both Truvada and Atripla since fourth-quarter 2016. Genvoya is now the company’s bestselling HIV product with a treatment-naïve patient share of 41%.
Gilead expects use of Truvada for pre-exposure prophylaxis (PrEP) to continue boosting sales of HIV franchise going forward, particularly in the U.S. Strong uptake for Truvada for use in the pre-exposure prophylaxis setting should also boost sales as the company saw a significant uptick in PrEP usage in 2017 with an estimated 136,000 patients using Truvada by the end of the second quarter.
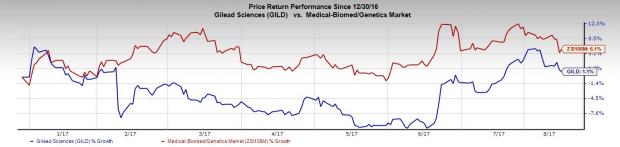
Meanwhile, Gilead’s stock has gained only 1.1% in the year so far, against the industry’s gain of 6.1%.
The uptake in HIV franchise should help the company combat the persistent decline in HCV franchise due to competitive pressure. The HCV business saw some improvement in the second quarter. The HCV franchise was boosted by the FDA approval of Vosevi. HCV patient starts in the first half were better than expectations but should see a gradual decline in the second half due to increased competition.
Harvoni, Sovaldi and Epclusa has being facing competition from AbbVie’s (NYSE:ABBV) Viekira Pak and Viekira XR and Bristol-Myers Squibb Company’s (NYSE:BMY) Daklinza. Competition as well as pricing pressure intensified further with the launch of Merck’s (NYSE:MRK) Zepatier.
Zacks Rank
Gilead currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
5 Trades Could Profit "Big-League" from Trump Policies
If the stocks above spark your interest, wait until you look into companies primed to make substantial gains from Washington's changing course.
Today Zacks reveals 5 tickers that could benefit from new trends like streamlined drug approvals, tariffs, lower taxes, higher interest rates, and spending surges in defense and infrastructure. See these buy recommendations now >>
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Merck & Company, Inc. (MRK): Free Stock Analysis Report
AbbVie Inc. (ABBV): Free Stock Analysis Report
Gilead Sciences, Inc. (GILD): Free Stock Analysis Report
Original post
Zacks Investment Research
