Galectin Therapeutics Inc (NASDAQ:GALT)
On Tuesday morning before the market opened, shares of Galectin Therapeutics plummeted more than 45% following negative trial results.
The company said their GR-MD-02 therapy did not meet its primary endpoint following the trial which is being developed to treat nonalcoholic steatohepatitis (NASH).
Galectin Therapeutics Technicals
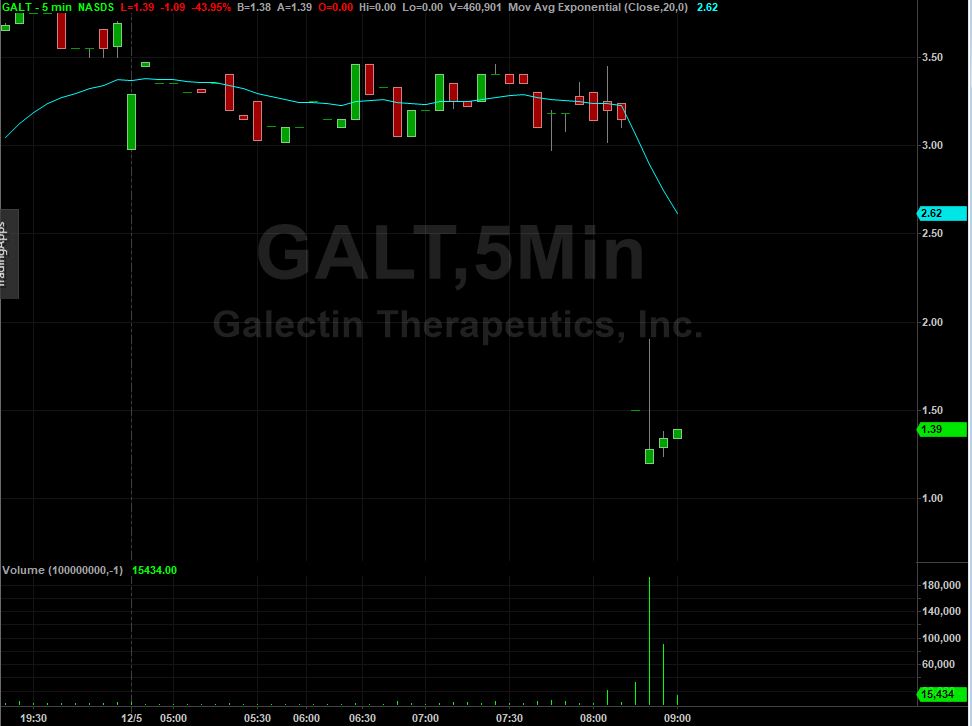
As you can see in the 5-minute chart above, shares of GALT got absolutely crushed following the trial results, which has pushed prices well below their 200-day moving average currently at $2.20.
Look for shares to be very volatile on elevated volume today with support not really coming in until the $1 level. Resistance will be met at $1.50, $1.60, $2 and $2.20.
Comments
“There is no current therapy for patients with NASH cirrhosis — and a therapy such as GR-MD-02 that could improve portal hypertension and potentially prevent the development of esophageal varices in NASH cirrhosis and subsequent complications — would be clinically valuable,” said Stephen A. Harrison, M.D., one of lead investigators of the NASH-CX trial, medical director of Pinnacle Clinical Research in San Antonio, Texas, and visiting professor of medicine at the University of Oxford, United Kingdom. “An indication of NASH cirrhosis without varices would be clinically meaningful to physicians, because it is standard of care for all patients with cirrhosis to have an upper endoscopy to assess for the presence of esophageal varices.”
Company Profile
Galectin Therapeutics, Inc., a clinical stage biopharmaceutical company, engages in the research and development of therapies for fibrotic disease and cancer.
The company’s lead product candidate includes galectin-3 inhibitor (GR-MD-02), a galactoarabino-rhamnogalacturonan polysaccharide polymer for the treatment of liver fibrosis and liver cirrhosis in non-alcoholic steatohepatitis patients, as well as for the treatment of cancer.
It also engages in developing GM-CT-01, which is in pre-clinical development state for the treatment of cardiac and vascular fibrosis, as well as focuses on developing GR-MD-02 for the treatment of psoriasis.
The company, through its collaborative joint venture, Galectin Sciences, LLC with SBH Sciences, Inc., is also involved in the research and development of small organic molecule inhibitors of galectin-3 for oral administration.
The company was formerly known as Pro-Pharmaceuticals, Inc. and changed its name to Galectin Therapeutics, Inc. in May 2011. Galectin Therapeutics, Inc. was founded in 2000 and is based in Norcross, Georgia.
