FibroGen Inc (NASDAQ:FGEN)
FibroGen, Inc. (FGEN), a national biopharmaceutical company yesterday announced positive top line results from its Phase 2 study of Pamrevlumab in patients with pulmonary fibrosis.
The study showed that pamrevlumab met its primary endpoint and showed statistical significant results compared to the placebo.
It was a double blind study that consisted of 103 patients that were randomly given the placebo or pamrevlumab during a 48 week period.
FibroGen, Inc. CMO’s Comments
“The positive results from this randomized placebo-controlled Phase 2 study build on our previous clinical data which demonstrated the potential of pamrevlumab to slow the progression of IPF with a good safety and tolerability profile,” said Peony Yu, M.D., FibroGen’s Chief Medical Officer. “We are conducting further analyses and look forward to presenting additional data from this study in the months ahead. We believe these results support a Phase 3 program in patients suffering from this debilitating and deadly disease.” Globe Newswire
FGEN Technical Analysis
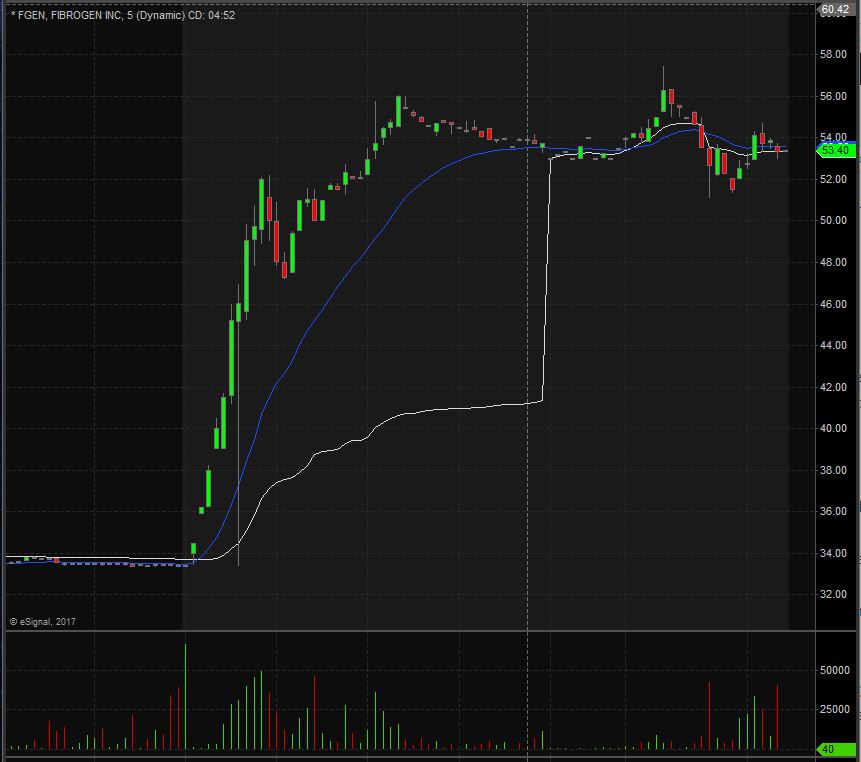
FGEN opened trading yesterday at $34.90 which was down from the previous day’s trading close of $35.00. FGEN closed trading yesterday at $33.40 and spiked up after hours to $53.95, equivalent to a 62% increase from the closing price. Taking a look at the daily chart we can see that we are in unchartered territory as with the spike up FGEN is now trading at all time highs.
Taking a closer look at the daily chart we can see that before the spike up FGEN had already been in an overall upward trend dating back to April 5th when it traded at $22.95. FGEN has a float of 64.9 million shares and traded 3.60 times the normal daily trading volume on Monday.
For trading purposes, I would like to see FGEN open trading on Tuesday above $47.00 and if it does I would be looking to take a long position at the bell. My stop loss would be $0.75 from my entry position fearing anything more than that and the stock would start to fill in the gap up.
Company Profile
FibroGen, Inc., a research-based biopharmaceutical company, discovers, develops, and commercializes therapeutic agents to treat serious unmet medical needs in the United States.
It is developing Roxadustat, an oral small molecule inhibitor of hypoxia inducible factor prolyl hydroxylases (HIF-PHs) that is in Phase III clinical development for the treatment of anemia in chronic kidney disease; Pamrevlumab, a human-monoclonal antibody that inhibits the activity of connective tissue growth factor, which is in Phase II clinical development for the treatment of idiopathic pulmonary fibrosis, pancreatic cancer, liver fibrosis, and Duchenne muscular dystrophy; and FG-5200 for the treatment of corneal blindness resulting from partial thickness corneal damage.
It has collaboration agreements with Astellas Pharma Inc. and AstraZeneca AB. The company was incorporated in 1993 and is headquartered in San Francisco, California.
