EyeGate Pharmaceuticals Inc (NASDAQ:EYEG)’s Ocular Bandage Gel (OBG) is progressing in its ongoing pivotal study in corneal wound healing following photorefractive keratectomy (PRK) surgery. The firm recently met its 75% recruitment target (planned n=260 patients) and data are expected by YE19. If positive, it should lead to a market approval filing in H120. EyeGate will shortly begin a follow-on pilot study in punctate epitheliopathies (PE), a much larger indication that is related to dry eye disease (DED). Prior OBG PRK studies showed improved wound healing and a prior PE pilot trial showed improvement in patient symptoms, which may bode well for OBG’s prospects.
OBG believed to improve wound healing
EyeGate’s OBG is a topical eye drop based on its proprietary, crosslinked thiolated carboxymethyl-hyaluronic acid (CMHA-S). OBG is intended to create a thin, durable and protective coating to a damaged or disrupted ocular surface, serving to facilitate corneal re-epithelization, and the crosslinking may resist degradation. In a 2018 PRK study (n=45), two OBG dosing arms were compared to the standard of care (SoC), a bandage contact lens (BCL) plus artificial tears. Both OBG arms outperformed the SoC; at day 3, 73% and 87% of eyes in the two OBG arms were healed vs 67% for SoC. These data were consistent with a pilot PRK study (n=39) completed in Q117, which also found OBG provided quicker wound closure vs SoC.
PE follow-on study to start shortly, data by YE19
EyeGate received FDA approval in August to begin a follow-on pilot study using OBG to treat patients with PE. In EyeGate’s 2018 OBG study (n=30) in subjects with PE compared to subjects receiving a commercial lubricant drop, OBG showed a statistically significant reduction in symptoms at day 7 and 28, and supporting data in the reduction of central corneal staining reduction (30% for OBG vs 14% for lubricant arm) at day 7. The firm plans to begin enrolment for the follow-up study in Q419, which will assess several endpoints in PE patients. Following pilot study data, expected by YE19, the firm plans to meet with the FDA to establish the most suitable endpoint to use in a pivotal study, planned to start in 2020. If all goes well, the firm plans to launch OBG in both PE and post-PRK wound healing in H221. The PE market is much larger than wound healing, as c 30 million Americans have DED-related symptoms, vs up to 2–3 million corneal injuries per year in the US.
Valuation: EV of $6.0m; funded into late Q120
EyeGate ended H119 with $4.47m cash and since raised $1.9m in equity. Its H119 operating burn rate was $3.57m, but H219 burn should increase given the ongoing and planned OBG studies. It estimates that cash on hand should be sufficient into late Q120 and we anticipate that an additional $5–9m may be needed to fund the firm through the completion of a PE pivotal study (expected by YE20).
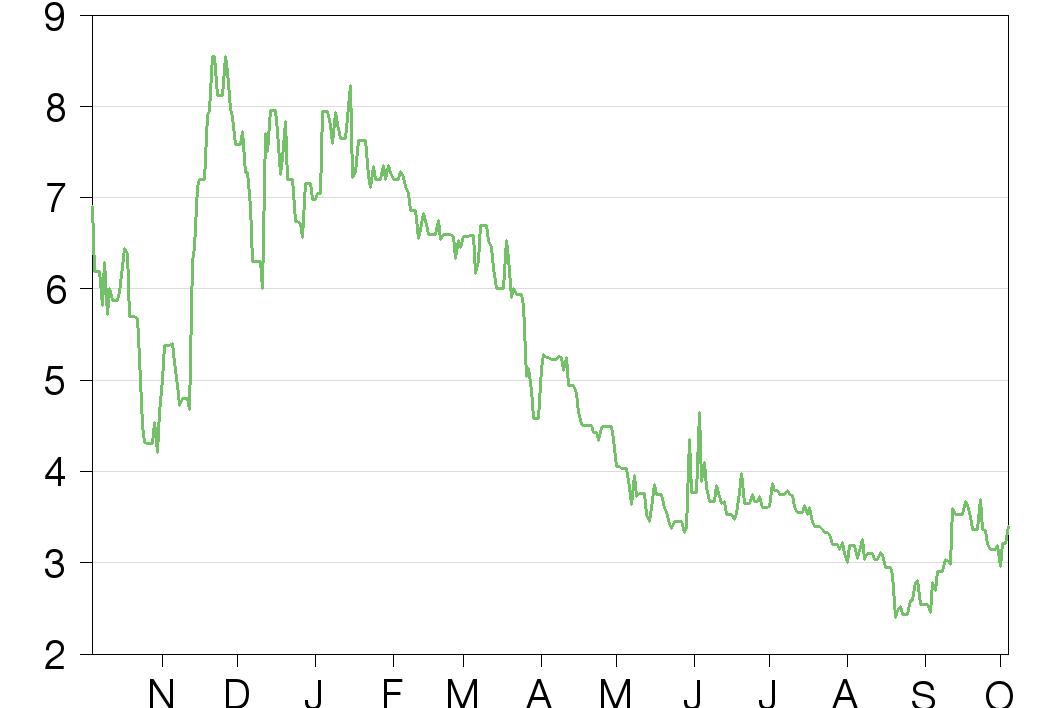
Historical financials
Share price graph
Business description
EyeGate Pharmaceuticals is a specialty pharma firm developing Ocular Bandage Gel (OBG), a clinical-stage proprietary topical eye drop formulation being advanced for moderate DED and corneal wound healing. EyeGate’s EGP-437 delivers a corticosteroid through an iontophoresis drug delivery system.