A solid grasp with Graspa
Erytech is positioned to join the select group of European biotechnology companies with a marketed product. Graspa targets frail and elderly leukaemia patients and is based on the well-established childhood leukaemia treatment L-asparaginase. Top-line pivotal data are anticipated in Q414, which should allow for first European launch in early 2016 with partner Recordati. We value Erytech at €96m, or €17.2/share.
A rush of blood
Graspa has been developed using Erytech’s unique technology, which can capture proteins within red blood cells (erythrocytes). This encapsulation protects the molecule from the body’s natural defences, and limits sudden exposure post conventional drug administration, which can have side effects. In addition, the encapsulated molecule’s half-life can be extended. These effects allow lower doses to achieve the same efficacy as standard/regular drug, while reducing side effects.
Graspa could be launched in 2016
Graspa is encapsulated L-asparaginase, a child leukaemia treatment that has been in use for over 30 years. However, its use in elderly and frail patients is limited owing to serious side effects and allergic reactions. Graspa has already demonstrated improved safety with equivalent efficacy compared to L-asparaginase and is being investigated in pivotal trials in both acute lymphoblastic leukaemia (ALL) and acute myeloid leukaemia (AML), with Phase III ALL data anticipated in Q414. This could allow for first European launches in 2016. Graspa is partnered with Recordati in Europe. Based on 16,000 ALL patients in the US and EU, and 40,000 AML patients, we forecast Graspa peak sales of €345m in 2025.
Broadening potential in solid tumours
Eryasp (Graspa) is also being developed in solid tumours sensitive to L-asparaginase. Phase II pancreatic cancer plans have recently been announced, although this is not included in our valuation owing to the earlier development stage. There were 45k pancreatic cancer patients diagnosed in the US in 2013.
Valuation: Risk-adjusted NPV of €96m
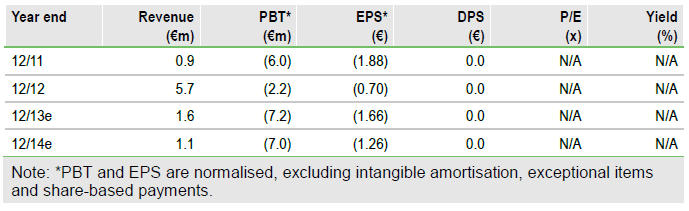
We value Erytech at €96m or €17.2/share based on a risk-adjusted NPV analysis, including net cash and Graspa in ALL and AML. Estimated €15.6m net cash at end September should be sufficient to fund operations to end 2015. Phase III ALL data and progress in AML and solid tumours will likely be key for valuation.
To Read the Entire Report Please Click on the pdf File Below