Eleven Biothera (NASDAQ:EBIO), a biopharmaceutical company yesterday announced that the IND application for their drug EBI-031 has become effective. The company is set to receive a $22.5 million milestone payment from F. Hoffman-La Roche.
Eleven Biotherapeutics, Inc. CEO’s Comments
We look forward to Roche advancing EBI-031 into the clinic to explore its potential use for ocular diseases, such as diabetic macular edema, in an effort to bring this potential treatment to patients, said Abbie Celniker, Ph.D., President and Chief Executive Officer of Eleven Biotherapeutics. Business Wire
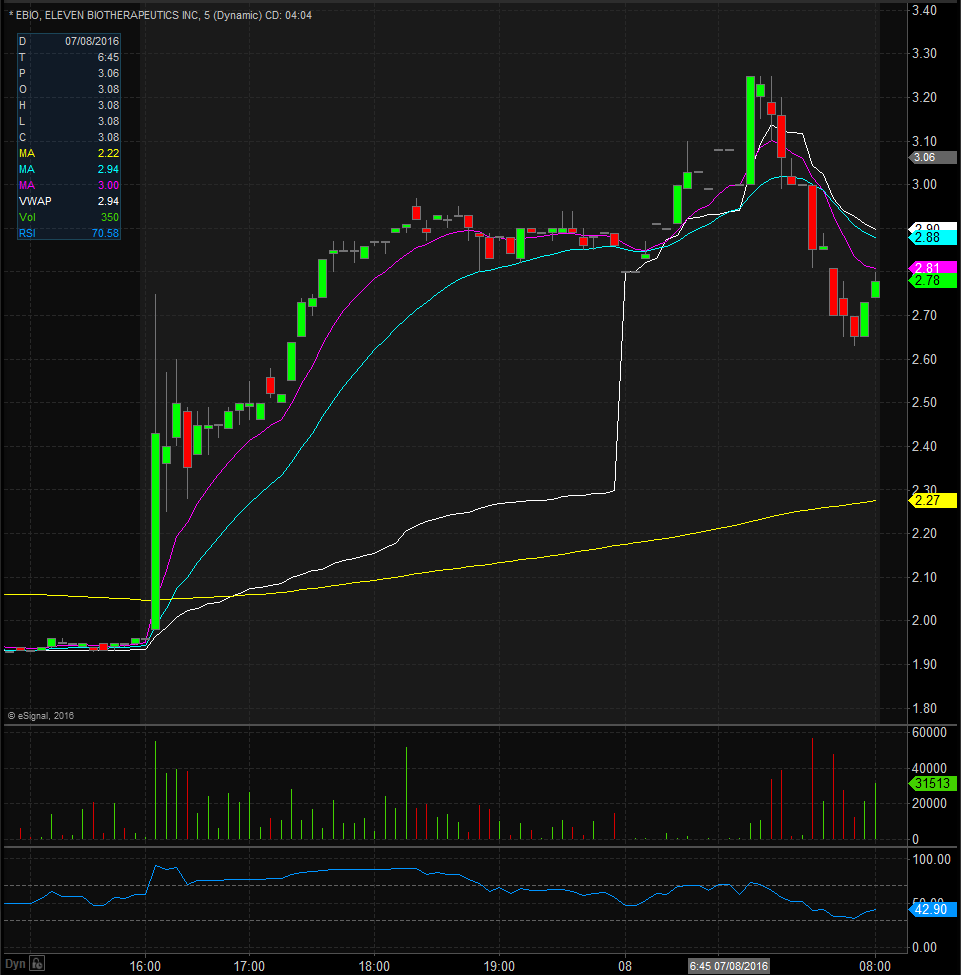
EBIO Technical Analysis
EBIO opened trading yesterday at $2.01, which was down from the previous days trading of $2.03. EBIO closed trading yesterday at $1.96 and spiked up after market to $2.90, equivalent to a 48% increase from the closing price. Taking a look at the daily chart we can see the last time EBIO traded above these levels was on December 31 when it traded at $3.01. Taking a closer look at the daily chart we can see that EBIO has been on an overall upward trend dating back to April 13th when it traded at $0.34. EBIO has a float of 5.56 million shares and traded below the normal daily trading volume on Thursday. For trading purposes, I would like to see EBIO open trading on Friday above $2.60 and if it does I would be looking to take a long position at the bell. My stop loss would be $0.15 from my entry position fearing anything more than that and the stock would start to fill in the gap up.
Company Profile
Eleven Biotherapeutics, Inc., incorporated on February 25, 2008, is a preclinical-stage biopharmaceutical company. The Company applies its AMP-Rx platform to the discovery and development of protein therapeutics to treat diseases of the eye. The Company’s product candidate, which is still in preclinical development, is EBI-031, which was designed, engineered and generated using its AMP-Rx platform and are developing as an intravitreal injection for diabetic macular edema (DME) and uveitis. The Company’s therapeutic approach is based on the role of cytokines in diseases of the eye, its understanding of the structural biology of cytokines and its ability to design and engineer proteins to modulate the effects of cytokines. Cytokines are cell signaling molecules found in the body that have inflammatory effects.
Its EBI-031 program is based on the role that elevated levels of the inflammatory cytokine interleukin-6 (IL-6) plays in the initiation and maintenance of the inflammation and pain associated with DME and uveitis. The Company designed and engineered EBI-031using its AMP-Rx platform to block two forms of IL-6: free IL-6 and IL-6 bound to soluble IL-6 receptor, or IL-6R. The Company is developing EBI-031 as an intravitreal injection for DME and uveitis. In addition to EBI-031, the Company has another product candidate in early preclinical development, which is designed to block vascular endothelial growth factor (VEGF). The Company is developing this product candidate as an intravitreal injection for the treatment of certain retinal diseases, such as wet age-related macular degeneration (AMD).