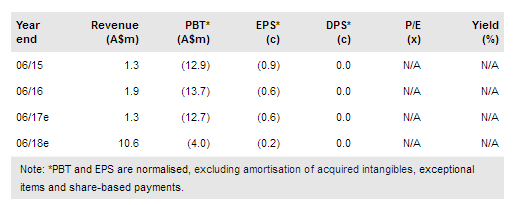
Preliminary efficacy data, which Prima BioMed Ltd ADR (NASDAQ:PBMD) presented at ASCO from the 15-patient, safety run-in phase of its AIPAC study, showed sustained immune activation and an encouraging 47% tumour response rate in breast cancer following IMP321 plus paclitaxel combination therapy. Recruitment in the randomised Phase IIb component of AIPAC is ongoing, with top-line data likely by mid-2019. Efficacy data from the final two cohorts in the TACTI-mel trial of IMP321 plus Keytruda in melanoma are expected in H217 and H118 respectively. The 47% AIPAC response rate is in line with Phase I studies and consistent with our expectations, so we leave our valuation unchanged at A$252m (12c per share).
Encouraging response rate in AIPAC run-in
The 47% response rate in 15 metastatic breast cancer patients treated with IMP321 in combination with paclitaxel during the safety run-in study was similar to the 50% response rate reported in a previous Phase I trial, and higher than the c 30% response rate seen in published studies of weekly paclitaxel in breast cancer. Top-line PFS data from the ongoing 226-patient, randomised Phase IIb component of AIPAC could mature sometime between late 2018 and mid-2019; patients receive either 30mg of IMP321 or placebo, in combination with weekly paclitaxel. IMP321 is a LAG-3-based, antigen-presenting cell activator that enhances immune responses.
To read the entire report Please click on the pdf File Below: