Celgene Corporation (NASDAQ:CELG) announced that the FDA has approved Inrebic (fedratinib) for the treatment of adult patients with intermediate-2 or high-risk primary or secondary (post-polycythemia vera or post-essential thrombocythemia) myelofibrosis.
The drug provides a new, once-daily oral option for patients affected by rare bone marrow cancer. Between 16,000 and 18,500 patients are estimated to be living with myelofibrosis and 1.5 of every 100,000 people are expected to be diagnosed with the same annually.
The approval was based on positive results from the JAKARTA2 study. The results of the study showed that 37% of patients treated with Inrebic 400 mg experienced a 35% or more reduction in spleen volume compared to just 1% (1 of 96) of patients who received placebo.
However, Inrebic, an oral kinase inhibitor with activity against wild type and mutationally activated Janus Associated Kinase 2 (JAK2) and FMS-like tyrosine kinase 3 (FLT3), carries a boxed warning for serious and fatal encephalopathy, including Wernicke’s.
The drug was added to Celgene’s portfolio through the acquisition of Impact Biomedicines in 2018.
Competition is stiff in this market. Incyte’s (NASDAQ:INCY) Jakafi, a first-in-class JAK1/JAK2 inhibitor, is already approved for intermediate or high-risk myelofibrosis.
Nevertheless, the approval will strengthen Celgene’s oncology portfolio. Approval of new drugs and label expansion of the existing ones will drive the top line.
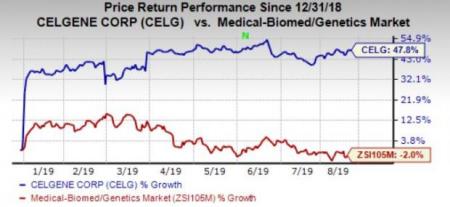
Celgene’s shares have soared 47.8% so far this year against the industry’s decline of 2%.
In July 2019, the FDA approved Otezla, an oral, selective inhibitor of phosphodiesterase 4 (PDE4), for the treatment of adult patients with oral ulcers associated with Behçet’s Disease. In May 2019, the European Commission (EC) approved a label expansion of lead drug Revlimid (lenalidomide) in combination with Velcade and dexamethasone (RVd) for the treatment of adult patients with previously-untreated multiple myeloma, who are not eligible for transplant. Also, during the same month, the FDA approved Revlimid in combination with a rituximab product for the treatment of adult patients with previously-treated follicular lymphoma (FL) or marginal zone lymphoma (MZL).
Meanwhile, the FDA accepted for review the new drug application (NDA) for ozanimod to treat patients with relapsing forms of multiple sclerosis (RMS) in the United States. The European Medicines Agency (EMA) also accepted for review the Marketing Authorization Application for the drug to treat adults with relapsing-remitting multiple sclerosis in the European Union.
The FDA also accepted the company’s Biologics License Application (BLA) for investigational erythroid maturation agent, luspatercept. The candidate is being developed in collaboration with Acceleron Pharma Inc. (NASDAQ:XLRN) to treat adult patients with very low to intermediate-risk myelodysplastic syndromes (MDS)-associated anemia, who have ring sideroblasts and require red blood cell (RBC) transfusions, and also those with beta-thalassemia-associated anemia, who require RBC transfusions.
Celgene has been in the news since January 2019 due to a merger announcement with large-cap pharma Bristol-Myers Squibb Co. (NYSE:BMY) for a whopping $74 billion.
Celgene currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
The Hottest Tech Mega-Trend of All
Last year, it generated $8 billion in global revenues. By 2020, it's predicted to blast through the roof to $47 billion. Famed investor Mark Cuban says it will produce "the world's first trillionaires," but that should still leave plenty of money for regular investors who make the right trades early.
See Zacks' 3 Best Stocks to Play This Trend >>
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Celgene Corporation (CELG): Free Stock Analysis Report
Incyte Corporation (INCY): Free Stock Analysis Report
Acceleron Pharma Inc. (XLRN): Free Stock Analysis Report
Original post
Zacks Investment Research
