One step forward, one step back
Can-Fite’s lead compound CF101 met its primary endpoint in a Phase IIb study in rheumatoid arthritis (RA), but was unsuccessful in meeting endpoints in the dry eye syndrome (DES) Phase III trial. We have increased our probability of commercial success in RA to 20% (from 15%) and have removed DES from our valuation, as the likelihood of commercial viability appears remote. Our new $90m rNPV (vs $99m previously) continues to reflect significant upside to the current EV of $40m. The next major catalyst in H114 could be a licensing agreement (US and/or Europe) for CF101, which would also help to determine the next clinical steps in RA.
Targeting A3AR patients pays off in RA
In the 12-week, 79-patient Phase IIb RA study, patients pre-selected with high A3 adenosine receptor (A3AR) expression showed a statistically significant (p=0.035) improvement vs placebo in the ACR20 clinical score, as well as ameliorations in ACR50 and ACR70 scores. The drug was also well tolerated and the study has provided proof-of-concept (POC) that CF101, an A3AR agonist, can potentially reduce signs and symptoms of inflammatory disease in this group of patients. We estimate that Can-Fite will conclude a US and/or European commercialisation agreement for CF101 before engaging in future RA studies. Can-Fite is planning an end-of-Phase II meeting with the FDA and intends to submit a Phase III protocol, although the next development step could also be a larger Phase IIb trial.
Not shedding a tear for CF101 in DES
In the 24-week placebo-controlled Phase III study for CF101 in DES (n=237), neither the 0.1mg nor the 1.0mg twice-daily treatment arms provided a statistically significant improvement in the primary efficacy endpoint of complete clearing of corneal staining, nor the secondary efficacy endpoints. Given the unsuccessful result and that DES is a challenging indication to demonstrate consistent efficacy, we now view the probability of commercial success for CF101 in DES as remote.
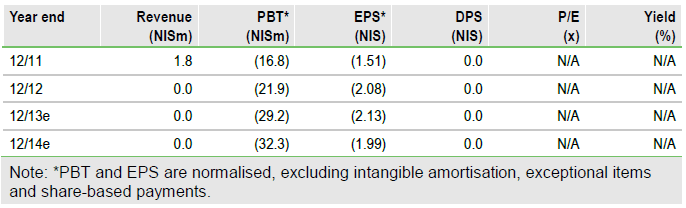
Valuation: rNPV lowered to $90m, $11.86/share basic
We have removed DES from our valuation, while raising our RA probability estimate to 20% (from 15%, previously) and we now value Can-Fite’s rNPV at $90m (or $11.86 per basic ADR, after including $6.9m estimated Q413 net cash). This compares favourably to the current EV of $40m. We estimate Can-Fite’s next major potential catalyst could be securing a CF101 licensing agreement in the US and/or Europe.
To Read the Entire Report Please Click on the pdf File Below