Bristol-Myers Squibb Company (NYSE:BMY) announced disappointing top-line results from the CheckMate-214 trial. The study investigated immuno-oncology drug Opdivo (nivolumab) in combination with Yervoy (ipilimumab) versus Pfizer Inc.’s (NYSE:PFE) Sutent in intermediate and poor-risk patients previously untreated advanced or metastatic renal cell carcinoma.
Although the combination showed an improvement in progression-free survival (PFS), it did not reach statistical significance as the median PFS was 11.56 months for the Opdivo and Yervoy combination versus 8.38 months for Sutent. Hence, the trial did not meet its primary endpoint. Nevertheless, the combination met the co-primary endpoint of objective response rate (ORR) and achieved a 41.6% ORR versus 26.5% for Sutent.
However, the company has decided to continue with the study as planned to allow the third co-primary endpoint of overall survival to mature. The tolerability profile observed in CheckMate-214 was consistent with that observed in previously reported studies of this dosing schedule.
We note that renal cell carcinoma (RCC) is the most common type of kidney cancer in adults and accounts for more than 100,000 deaths worldwide each year. Among these, clear-cell RCC is the most prevalent type of RCC and constitutes 80-90% of all cases thereby underlying the demand for the same.
Bristol-Myers received a huge boost when its high-profile immuno-oncology drug, Opdivo, became the first PD-1 immune checkpoint inhibitor to gain regulatory approval anywhere in the world in Jul 2014. It is currently approved in several countries including the U.S., the EU and Japan for several cancer indications.
However, the company has had its share of pipeline and regulatory setbacks. In Oct 2016, Bristol-Myers announced the final primary analysis of CheckMate-026, a phase III study that evaluated the use of Opdivo monotherapy as a first-line treatment of patients with advanced non-small-cell lung cancer (NSCLC) whose tumors expressed PD-L1 greater than or equal to 1%.
In Jan 2017, the company announced not to pursue accelerated regulatory pathway for the regimen of Opdivo plus Yervoy in first-line lung cancer in the United States, based on a review of available data. Bristol-Myers’ failed efforts to expand Opdivo’s label to include first-line treatment of lung cancer adversely impacted shares.
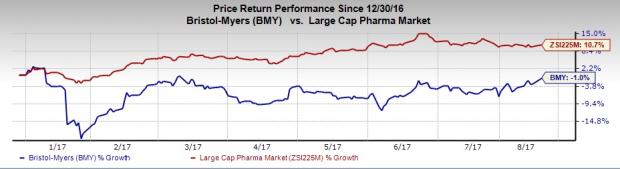
Bristol-Myers’ stock has declined 1% in the year so far, against the industry’s 10.7% gain.
Opdivo faces stiff competition from Merck &Co’s (NYSE:MRK) Keytruda and Roche Holding’s (OTC:RHHBY) Tecentriq. The recent FDA approval of Merck’s Keytruda for the first-line treatment of metastatic nonsquamous NSCLC will further impact sales.
Moreover, with the failure of AstraZeneca’s (NYSE:AZN) Mystic study on Imfinzi in NSCLC, investors are worried about the outcome of the CheckMate-227 study on Opdivo for first-line NSCLC given the similarity of trials. While management has played down the uncertainty regarding the trial, concerns remain.
Zacks Rank
Bristol-Myers currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
The Hottest Tech Mega-Trend of All
Last year, it generated $8 billion in global revenues. By 2020, it's predicted to blast through the roof to $47 billion. Famed investor Mark Cuban says it will produce "the world's first trillionaires," but that should still leave plenty of money for regular investors who make the right trades early.
See Zacks' 3 Best Stocks to Play This Trend >>
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Astrazeneca PLC (LON:AZN): Free Stock Analysis Report
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Merck & Company, Inc. (MRK): Free Stock Analysis Report
Original post
Zacks Investment Research
