Bristol-Myers Squibb Company (NYSE:BMY) announced that the FDA has approved Orencia for the treatment of adults with active psoriatic arthritis (PsA).
The approval was based on results from two randomized, double-blind, placebo-controlled trials. Per the data from the trials, Orencia improved (or reduced) disease activity in both TNF-naive and exposed patients suffering from increased disease activity, high tender and swollen jointsfor more than seven years.
Orencia is already indicated for adult patients with moderate to severe active rheumatoid arthritis (RA)as well as for reducing signs and symptoms in pediatric patients with moderately to severely active polyarticular juvenile idiopathic arthritis.
Sales of Orencia came in at $535 million in the first quarter of 2017, up from $475 million in the year-ago quarter. U.S. sales in the quarter were up 13% despite unfavorable inventory movement. However, demand was softer in the subcutaneous market in the beginning of the year due to early coverage resets.
A label expansion of the drug will further boost sales.
We note that in Sep 2016, Orencia became the first biologic therapy to gain EU approval specifically for the treatment of methotrexate (MTX)-naïve RA patients with highly active and progressive disease.
Competition is stiff in the psoriatic arthritis with the presence of drugs like AbbVie’s (NYSE:ABBV) Humira and Novartis’ (NYSE:NVS) Cosentyx.
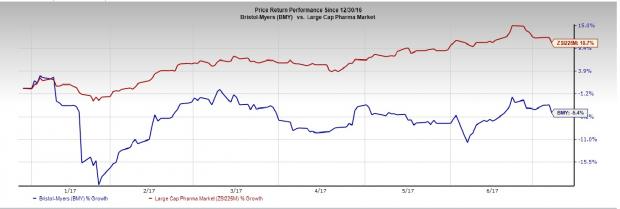
While Bristol-Myers’ share price has declined 5.4% year to date, the Zacks classified Large Cap Pharmaceuticals industry has gained 10.7%.
Bristol-Myers’ key products – Opdivo, Orencia, Eliquis and Sprycel – should continue to fuel the company’s top line. Meanwhile, Bristol-Myers is working on expanding the label of Opdivo which should boost performance further. The EC recently approved Opdivo as a monotherapy for the treatment of SCCHN in adults. However, Opdivo is facing competitive challenges in the U.S. With the FDA approving Merck’s (NYSE:MRK) Keytruda, for the first-line treatment of metastatic nonsquamous NSCLC, the company is expected to see further loss of market share.
Zacks Rank
Bristol-Myers currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank stocks here.
Will You Make a Fortune on the Shift to Electric Cars?
Here's another stock idea to consider. Much like petroleum 150 years ago, lithium power may soon shake the world, creating millionaires and reshaping geo-politics. Soon electric vehicles (EVs) may be cheaper than gas guzzlers. Some are already reaching 265 miles on a single charge.
With battery prices plummeting and charging stations set to multiply, one company stands out as the #1 stock to buy according to Zacks research. It's not the one you think.
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Novartis AG (NVS): Free Stock Analysis Report
Merck & Company, Inc. (MRK): Free Stock Analysis Report
AbbVie Inc. (ABBV): Free Stock Analysis Report
Original post
Zacks Investment Research
