Bristol-Myers Squibb Company (NYSE:BMY) announced that the FDA has accepted and granted a priority review designation to the supplemental Biologics License Application (sBLA) for the label expansion of Opdivo (nivolumab) to patients with hepatocellular carcinoma (HCC), after prior Nexavar therapy. HCC is the most common type of liver cancer.
The submission was based on data from the phase I/II CheckMate 040 study investigating Opdivo in advanced HCC patients with and without hepatitis B virus or hepatitis C virus infections. The FDA is expected to give its decision on Sep 24, 2017.
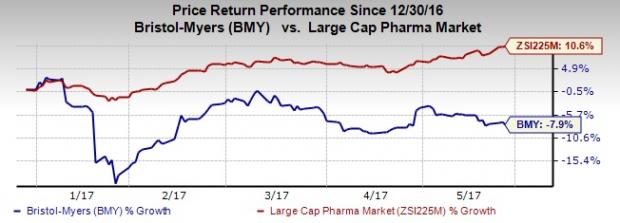
Bristol-Myers’ shares have lost 7.9% year to date against the Zacks classified Large Cap Pharmaceuticals industry’s gain of 10.6%.
We note that the drug is already approved for varied indications in both the EU and the U.S. Also, it received approvals for several indications including melanoma, head and neck, lung, kidney and blood cancer. In fact, the Opdivo+Yervoy regimen is approved in multiple markets for the treatment of melanoma.
Opdivo generated revenues of $1.13 billion in the first quarter of 2017, up 60% from the year-ago period.
In Apr 2017, Bristol-Myers announced a collaboration agreement with clinical-stage biopharmaceutical company Apexigen, Inc. Moving ahead, both the companies will collaborate for a clinical trial to evaluate Bristol-Myers Squibb’s immuno-oncology drug Opdivo in combination with Apexigen’s APX005M on patients with advanced solid tumors.
Earlier, the company had announced that the Committee for Medicinal Products for Human Use (CHMP) has recommended the approval of immunotherapy Opdivo as monotherapy for the treatment of squamous cell cancer of the head and neck (SCCHN) in adults progressing on or after platinum-based therapy in March. The European Commission will now review the CHMP recommendation.
The positive opinion from the CHMP was based on results from open-label, randomized phase III trial, CheckMate -141, that evaluated the overall survival (OS) of Opdivo in previously treated patients with SCCHN following platinum-based therapy compared to investigator’s choice of therapy (methotrexate, docetaxel, or cetuximab) in the adjuvant, primary, recurrent or metastatic setting. The trial was stopped early in Jan 2016 based on a planned interim anlaysis as assessed by the independent Data Monitoring Committee.
Zacks Rank and Other Key Picks
Bristol-Myers currently carries a Zacks Rank #3 (Hold). Some other better ranked stocks in the health care sector are VIVUS, Inc. (NASDAQ:VVUS) , MEI Pharma, Inc. (NASDAQ:MEIP) and Aeglea BioTherapeutics (NASDAQ:AGLE) . While VIVUS and MEI Pharma sport a Zacks Rank #1 (Strong Buy), Aeglea carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
VIVUS’ loss per share estimates have narrowed from 50 cents to 39 cents for 2017 over the last 60 days. The company delivered positive earnings surprises in all the four trailing quarters, with an average beat of 233.69%.
MEI Pharma’s estimates have turned around from loss per share of 1 cent to gain of 1 cent per share for 2017 over the last 60 days. The company came up with positive earnings surprises in three of the four trailing quarters, with an average beat of 66.56%.
Aeglea’s loss per share estimates have narrowed from $3.64 to $2.48 for 2017 over the last 60 days. The company pulled off positive earnings surprises in three of the four trailing quarters, with an average beat of 20.75%.
Looking for Stocks with Skyrocketing Upside?
Zacks has just released a Special Report on the booming investment opportunities of legal marijuana.
Ignited by new referendums and legislation, this industry is expected to blast from an already robust $6.7 billion to $20.2 billion in 2021. Early investors stand to make a killing, but you have to be ready to act and know just where to look. See the pot trades we're targeting>>
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
VIVUS, Inc. (VVUS): Free Stock Analysis Report
Aeglea BioTherapeutics, Inc. (AGLE): Free Stock Analysis Report
MEI Pharma, Inc. (MEIP): Free Stock Analysis Report
Original post
Zacks Investment Research