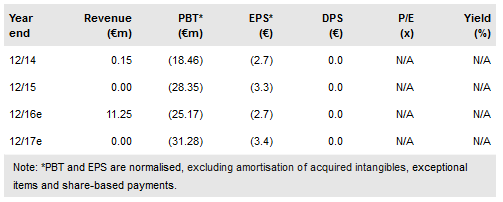
Celyad SA (NASDAQ:CYAD) has enrolled the first patient the Phase Ib THINK study. The THINK Phase Ib trial is a major expansion of CAR therapy with five solid tumours plus AML and MM being explored. The first patient has colorectal cancer, a key move into solid tumours, and will be dosed at 3 x 108 autologous cells. In the previous Phase I study, one patient at the highest 3 x 107 dose showed unexpected signs of efficacy. The US allogenic CAR patent has been confirmed. Our interim indicative value remains at €45 per share.
NKR-2 has a lead positon in solid tumours
Celyad has started the Belgian arm of the immuno-oncology autologous NKR-2 trials (THINK). US approval of the trial is expected soon. THINK recruits patients with two haematological and five solid tumours. Celyad has enrolled the first patient at a single dose of 3 x 108. Once this dose cohort completes, the dose rises to 1 x 109 then 3 x 109 cells. Celyad then aims to treat about 14 patients per tumour type at the highest dose. The expansion phase may start in H217, with six-month results possible in H218. The exploration of NKR-2 in solid tumours puts Celyad in a leading position in this area. Other CAR companies will initially have to compete for a limited number of patients in the congested CD19 area. There are a few, limited clinical trials using CAR constructs in solid tumours, but otherwise this large potential market is not being addressed by active clinical development.
To read the entire report Please click on the pdf File Below