BioMarin Pharmaceutical Inc. (NASDAQ:BMRN) announced that the FDA is currently not planning to hold an advisory committee meeting (AdCom) to discuss the Biologics License Application (BLA) for pipeline candidate, pegvaliase.
The company is looking to get pegvaliase approved for lowering blood phenylalanine (Phe) levels in adult patients with phenylketonuria (PKU), a rare genetic enzyme deficiency disorder, having uncontrolled blood Phe levels with existing treatment options.
Notably, the FDA responded to the company’s BLA through a Day-74 filing letter, communicating potential review issues. However, we are positive on the FDA’s current decision as it indicates the agency’s comfort with the safety and efficacy data of pegvaliase submitted from completed clinical studies. Also, the preliminary evaluation of the FDA is in line with the company’s expectations.
Additionally, an application in the EU for pegvaliase is expected to be filed by the end of this year.
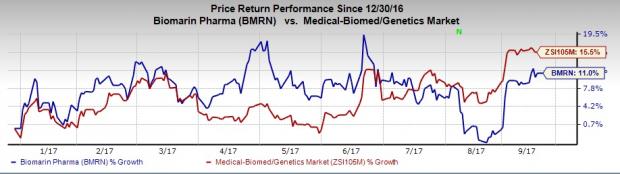
BioMarin’s shares are up 11% so far this year, comparing unfavorably with the 15.5% rally of the industry during the period.
We remind investors that in August, the FDA accepted the BLA for pegvaliase under priority review. However, the regulatory body requested additional information on chemistry, manufacturing and controls related to pegvaliase. The company expects submission of this information to the FDA to be classified as a major amendment that will extend the review process by three months. As of now, a decision is expected on Feb 28, 2018.
BioMarin is hoping that pegvaliase, if approved, will supplement the company’s phenylketonuria portfolio, also including Kuvan. A potential approval will strengthen the company’s commercial leadership in the treatment of PKU with potential to drive the company’s revenues, going forward.
However, Kuvan is set to face generic competition from 2020 onward. The approval of pegvaliase will help BioMarin offset any loss of revenues from Kuvan in the long term.
Per the company provided information, approximately 50,000 individuals in the developed countries are diagnosed with PKU, representing a significant need for such therapies.
We remind investors that in January last year, BioMarin acquired all global rights to Kuvan and pegvaliase from Merck (NYSE:MRK) Serono. Previously, the company had exclusive rights to Kuvan in the United States as well as Canada and to pegvaliase in the United States plus Japan.
With this transaction, BioMarin has gained exclusive worldwide rights to Kuvan and pegvaliase with the exception of Kuvan in Japan. The acquisition of PKU rights in these markets represents a significant opportunity for BioMarin to establish commercial leadership for treatment of PKU, first with Kuvan and then with pegvaliase, upon regulatory developments.
Zacks Rank & Stocks to Consider
BioMarin currently carries a Zacks Rank #3 (Hold). Some better-ranked stocks in the pharma sector are Akebia Therapeutics, Inc. (NASDAQ:AKBA) , Aduro Biotech, Inc. (NASDAQ:ADRO) and ACADIA Pharmaceuticals Inc. (NASDAQ:ACAD) , all three carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Akebia’s loss per share estimates narrowed from $4.14 to $3.85 for 2017 and from $1.98 to $1.88 for 2018 over the last 60 days. Its share price soared 66.2% so far this year.
Aduro Biotech’s loss per share estimates reduced from $1.46 to $1.32 for 2017 and from $1.41 to $1.24 for 2018 over the last 60 days. The company delivered positive surprises in two of the trailing four quarters with an average beat of 2.53%.
ACADIA’s loss per share estimates narrowed from $2.82 to $2.57 for 2017 and from $2.07 to $1.90 for 2018 over the last 60 days. The company came up with positive earnings surprises in two of the last four quarters with an average beat of 7.97%.
New Report: An Investor’s Guide to Cybersecurity
Cyberattacks have become more frequent and destructive than ever. In fact, they’re expected to cause $6 trillion per year in damage by 2020.
The cybersecurity industry is expanding quickly in response to these threats. In fact, a projected $170 billion per year will be spent to protect consumer and corporate assets. Zacks has just released Cybersecurity: An Investor’s Guide to Locking Down Profits which reveals 4 promising investment candidates.
BioMarin Pharmaceutical Inc. (BMRN): Free Stock Analysis Report
Aduro Biotech, Inc. (ADRO): Free Stock Analysis Report
ACADIA Pharmaceuticals Inc. (ACAD): Free Stock Analysis Report
Akebia Therapeutics, Inc. (AKBA): Free Stock Analysis Report
Original post
Zacks Investment Research