Mental health disorders affect approximately one in eight people worldwide, totaling around 970 million individuals. In the United States, the situation is even more severe, with one in five adults—about 70 million people, experiencing some form of mental illness. Additionally, research co-led by Harvard Medical School indicates that nearly half of the global population will encounter a mental health disorder at some point in their lives. This highlights the urgent need for better psychiatric care, innovation, and support.
Effective management typically involves a combination of medications and psychotherapy for the best results. Commonly prescribed antidepressant medications include selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and atypical antidepressants, which help regulate mood by balancing neurotransmitter levels in the brain. In recent decades, pharmaceutical companies have introduced several blockbuster medications, each achieving billions of dollars in annual sales at their peak. The success of these drugs highlights both the vast patient population affected by depression and the critical need for effective pharmacological treatments.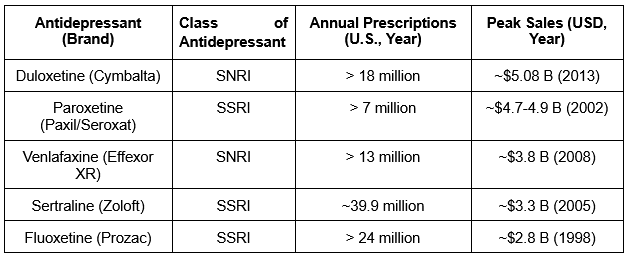
Exhibit 1: Select Blockbuster Antidepressant Drugs and Their Peak Sales
Amid this global mental health challenge, Bausch Health Companies (NYSE:BHC) faces both significant financial headwinds and a unique market opportunity to transform patient care with a breakthrough therapy for treatment-resistant depression (TRD).
Treatment-Resistant Depression: A Profound Unmet Medical) Need
Despite their widespread use, antidepressant medications are not without their flaws, leaving many medical needs unaddressed. A significant number of individuals diagnosed with depression do not achieve adequate relief from their symptoms, even when receiving appropriate treatment. This condition is known as treatment-resistant depression (TRD) and occurs when patients do not respond sufficiently after trying at least two different antidepressant therapies at effective dosages and durations. Approximately 30% of people treated for depression fall into this category, indicating the limitations of existing pharmacological options. This amounts to over 100 million people worldwide.
TRD can severely impact the quality of life, increase healthcare utilization, and raise the risks of disability and suicide. In the United States, around 2.8 million adults experienced medication-treated TRD within a 12-month period, resulting in an estimated annual economic burden of $43.8 billion. Additionally, traditional antidepressants often come with considerable side effects, delayed onset of therapeutic action, and variability in patient response, further complicating treatment outcomes.
Treatment-resistant depression presents a significant challenge due to its diverse nature and the complex interactions among biological, psychological, and social factors that affect treatment outcomes. While various therapeutic approaches are available, each option has specific limitations that restrict its widespread use:
- Pharmacological strategies often involve enhancing standard antidepressants with atypical antipsychotics like aripiprazole or quetiapine; however, these can lead to side effects such as weight gain and akathisia. Neuromodulation techniques, including electroconvulsive therapy (ECT) and transcranial magnetic stimulation (TMS), provide alternatives but are limited by factors such as invasiveness, accessibility, and inconsistent efficacy.
- In 2019, the U.S. Food and Drug Administration (FDA) approved Spravato (esketamine), which offers rapid relief of depressive symptoms. However, it necessitates careful medical supervision because of potential dissociative effects, risks of misuse, and concerns about its long-term safety and the sustainability of its therapeutic benefits. Psychotherapeutic interventions such as cognitive-behavioral therapy (CBT) can be helpful but may be less effective in severe cases or limited by availability.
- Emerging therapies, including psychedelic-assisted treatments, show potential but require further research to confirm their safety and efficacy.
Current pharmacological treatments for TRD are limited by safety profiles, practical applications, and overall effectiveness. Moreover, there are no FDA-approved oral monotherapies consisting of a single agent for TRD, which highlights a substantial treatment gap and emphasizes the urgent need for innovative, effective, and well-tolerated oral medications.
A Recent Breakthrough Brings New Hope
Bupropion, a norepinephrine-dopamine reuptake inhibitor (NDRI), is commonly prescribed for the treatment of major depressive disorder (MDD) and seasonal affective disorder (SAD). Initially approved by the FDA in 1985 as bupropion hydrochloride (Bup-HCl) and marketed under the brand name Wellbutrin, it introduced a novel mechanism by inhibiting the reuptake of norepinephrine and dopamine, distinguishing it from traditional antidepressants. While effective, Bup-HCl has been associated with side effects such as dry mouth, nausea, constipation, insomnia, anxiety, tremors, and excessive sweating. Bupropion hydrobromide (Bup-HBr), commercially known as Aplenzin, was developed and approved by the FDA in 2008 as a pharmacokinetic bioequivalent to Bup-HCl, offering a different salt form of bupropion. Marketed by Bausch Health Companies, Aplenzin remains under patent protection, with exclusivity set to expire in mid-2026.
Both Wellbutrin and Aplenzin are FDA-approved for treating MDD and SAD. In clinical practice, Wellbutrin is often prescribed off-label for TRD, either alone or in combination with other antidepressants. On the other hand, Aplenzin has been recently discovered to show the potential for enhanced tolerability and efficacy in treating TRD due to its unique pharmacodynamic profile.
At the 2024 NEI Scientific Congress, researchers Maxwell Zachary Price, BA, and Richard Louis Price, MD, presented a significant study comparing two forms of bupropion in patients with treatment-resistant depression. The study involved 30 outpatients who had an average PHQ-9 score of 20.9, indicating severe depression and a history of failing approximately four different antidepressant regimens. These participants were transitioned directly from generic extended-release bupropion hydrochloride (Wellbutrin) to an equivalent dosage of extended-release bupropion hydrobromide (Aplenzin).
Remarkably, within just two weeks of switching to the hydrobromide formulation (Aplenzin), patients reported a dramatic improvement in their depressive symptoms, with mean PHQ-9 scores dropping to 6.4, which is consistent with mild depression. Additionally, common side effects associated with bupropion hydrochloride, such as insomnia, anxiety, and gastrointestinal discomfort, were significantly reduced. As a result, 96.7% of participants chose to continue using the hydrobromide formulation.
The researchers propose that these superior clinical outcomes may be due to inherent pharmacodynamic differences between the two salt forms. Specifically, the bromide salt may more effectively enhance inhibitory GABA-A receptors. Its increased acidity might also improve blood-brain barrier permeability, boost tyrosine hydroxylase activity to elevate dopamine synthesis, and intensify monoamine oxidase inhibition.
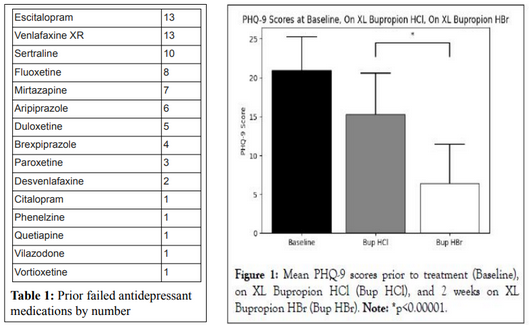
Exhibit 2: Clinical Study Results Comparing Bup Hbr to Bup HCl in Patients with TRD. Source: NEI Poster Presentation
These results challenge the common belief that pharmacokinetic bioequivalence in the blood ensures the same therapeutic performance pharmacodynamically in the brain, indicating that Bup-HBr (Aplenzin) might be a more effective and better-tolerated option for patients with TRD. While the clinical landscape highlights an urgent unmet need, Bausch Health’s own financial challenges accent the stakes of capturing this market opportunity.
A Potential Solution for Bausch’s Financial Challenges
Recent earnings reports have shown promising improvements at Bausch Health, with consecutive quarters of revenue growth and narrowed net losses. However, these gains are tempered by a heavy debt burden of approximately $21 billion and persistent investor concerns stemming from past financial and legal challenges. In this challenging environment, repositioning Aplenzin for treatment-resistant depression might emerge as not just a clinical opportunity but a strategic financial lever.
Bausch Health markets both Wellbutrin (the branded version of generic bupropion HCl) and Aplenzin (the only available formulation of bupropion HBr), which are approved for similar conditions, namely major depressive disorder (MDD) and seasonal affective disorder (SAD). In 2022, the total prescription volume for bupropion—covering bupropion HCl, bupropion HBr, and other formulations—was estimated at around 25 million annually in the United States, with Wellbutrin accounting for most of these prescriptions. Wellbutrin has been on the market for over 30 years and once achieved blockbuster status, with peak global sales exceeding $2 billion. In contrast, Aplenzin’s annual sales are much lower, totaling approximately $100 million. This disparity may be attributed to Aplenzin’s higher price point in a market dominated by less expensive generic alternatives. Additionally, Aplenzin has not received the same level of marketing support as Wellbutrin, further limiting its adoption among prescribers and patients. Before the Price & Price study, the first clinical study comparing Aplenzin to bupropion HCl, Aplenzin was regarded as a branded bioequivalent to generic bupropion HCl.
Aplenzin’s role as a treatment for MDD and SAD does not align optimally with market demand, leading to underutilization of its significant potential. However, recent findings by Dr. Maxwell Zachary Price and Dr. Richard Louis Price, MD, suggest that Aplenzin’s unique pharmacodynamic, efficacy, and tolerability profile may provide enhanced benefits for individuals with treatment-resistant depression (TRD). This observation indicates that instead of serving as a general market antidepressant, Aplenzin might achieve greater success if repositioned to target a more specific and underserved patient population. These research insights are particularly significant for a financially challenged Bausch Health, as they present a strategic opportunity to re-market Aplenzin for treatment-resistant depression, potentially unlocking additional value by meeting a critical unmet need while enhancing overall revenue and patient outcomes.
In an ideal scenario, Bausch would initiate clinical trials to expand Aplenzin’s approval for TRD, which could unlock significant market value. However, the company faces financial constraints that limit its ability to pursue this investment-intensive strategy. Another monetization approach Bausch could consider is out-licensing the Aplenzin asset and its associated contracts to a partner such as AbbVie (NYSE:ABBV) or Bristol-Myers Squibb (NYSE:BMY) while retaining manufacturing royalties. With expedited FDA review for TRD and the well-established safety profile of bupropion, only short-term studies would be necessary to confirm Aplenzin’s advantages over generic bupropion HCl.
One proposed study would involve 100 TRD patients who are randomized to receive either generic XL bupropion HCl or Aplenzin, with the primary endpoint being the change in MADRS score at six weeks. A second, double-blinded withdrawal study could have half of the participants continue on Aplenzin while the other half switch to a placebo.
Furthermore, the current set of patents for Aplenzin is valid until mid-2026, providing just enough time for research and FDA approval for TRD. Current Bausch patents would be evergreened by a 20-year U.S. patent for bupropion HBr in TRD, pending international approval, thereby, enabling a lengthy patent extension for the next two decades. This extension would cover the entire label, protecting it from "skinny labeling" and potential off-label use. Importantly, several federal circuit court and Supreme Court rulings involving medications such as Xifaxan, Coreg, and Vascepa have upheld that obtaining a new approved use can extend patent protection over the entire label, effectively shielding the product from generic carve-outs.
Aplenzin for Treatment-Resistant Depression: A $3 Billion Market Opportunity
TRD remains a significant unmet need, with no FDA-approved, self-administered oral monotherapy options that are both effective and safe. Existing treatments often fall short, leaving many patients without sufficient relief. This gap highlights the clinical challenges of managing TRD while also creating a considerable market opportunity for drugs like Aplenzin, which could offer improved efficacy and safety along with ease of administration. With 2.8 million patients in the United States alone, Aplenzin could potentially achieve blockbuster status by addressing just a small fraction (5%-10%) of the patient population.
Spravato (esketamine) nasal spray, developed by Johnson & Johnson (NYSE:JNJ), was approved in February 2019 as a combination therapy and received approval in January 2025 as a monotherapy for TRD. Notably, Spravato must be administered in a certified healthcare setting where patients are closely monitored for potential side effects such as sedation, dissociation, respiratory depression, and elevated blood pressure. Despite these restrictions, Spravato achieved blockbuster status in 2024, generating $1.08 billion in revenue and being administered to roughly 140,000 patients to date. J&J expects even higher sales, projecting that peak revenues could reach $5 billion.
In comparison, if Aplenzin is approved as the only self-administered oral monotherapy for TRD, it would offer a more convenient and accessible treatment alternative, potentially capturing a significant share of the 2.8 million U.S. TRD patients. Assuming a peak market share of 7%, an average monthly treatment cost of $2,500 per patient, and an average treatment duration of 6 months, the potential revenue from just the U.S. market could reach approximately $3 billion. These estimates are conservative and do not account for potential expansion into global markets. Additionally, despite Spravato’s strict administration requirements and associated side effects, it is projected to achieve up to $5 billion in peak sales. Given Aplenzin’s potential as a self-administered oral monotherapy without such restrictions, it is reasonable to assume that Aplenzin could generate revenue on par with, or even exceeding, Spravato’s projected peak sales.
With ongoing efforts to refinance debt and unlock value through strategic moves such as the spin-off of its eye-care division (Bausch + Lomb), Bausch Health is actively seeking new revenue streams. The emerging opportunity in treatment-resistant depression (TRD) not only promises significant clinical impact but could also serve as a catalyst for financial stabilization and growth. If repositioned successfully for TRD, Aplenzin could generate blockbuster revenues that address an urgent clinical need while alleviating Bausch Health’s financial liabilities and reinvigorating its market standing. Notably, with Bausch currently trading at a forward P/E ratio of around 1.5 (well below the industry average of 15 to 20 seen among its key competitors), a successful TRD strategy could trigger a significant re-rating, unlocking substantial investor value.
