AVEO Pharmaceuticals, Inc. (NASDAQ:AVEO) announced that the phase III study, TIVO-3, evaluating tivozanib in patients with refractory advanced renal cell carcinoma (RCC) has reached its enrollment target of 322 patients, two months earlier than the targeted timeline.
Top-line data from the study is expected in the first quarter of 2018.
The study is designed to compare the candidate with Bayer AG’s (OTC:BAYRY) Nexavar (sorafenib) for the third-line treatment of patients with refractory RCC. The study is evaluating patients with recurrent or metastatic RCC, who have failed at least two prior regimens including VEGFR-TKI therapy (other than Nexavar). In Feb 2017, the study successfully completed the first safety review by Safety Monitoring Committee (SMC).
The trial is being conducted to address the overall survival concerns from the TIVO-1 trial, identified by the FDA in the complete response letter issued in Jun 2013. The TIVO-3 trial, along with the previously completed TIVO-1 trial, is also intended to support an application for regulatory approval of tivozanib in the U.S. as a third-line treatment and as a first-line treatment for RCC.
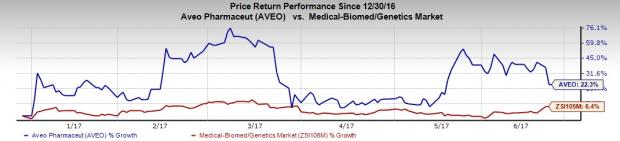
Shares of AVEO Pharma have outperformed the Zacks classified Medical-Biomed/Genetics industry so far this year. The stock has gained 22.3% compared with the broader industry’s increase of 6.4%.
In May 2017, the company completed its oral explanation to the Committee for Medicinal Products for Human Use (CHMP) as part of the review process for marketing authorization application (MAA) in the EU for tivozanib in first-line RCC. With the completion of oral explanation, it is expected that CHMP will provide an opinion soon. A positive opinion will boost the chances of its approval in 28 countries of EU.
Moreover, another phase II study is evaluating the candidate in combination with Bristol-Myers Squibb Company’s (NYSE:BMY) Opdivo in patients with advanced RCC.
Exelixis, Inc.’s (NASDAQ:EXEL) Cabometyx was approved in the U.S. and EU last year for the treatment of advanced RCC and is currently marketed in the U.S. Exelixis has planned to submit a supplemental New Drug Application (sNDA) for cabozantinib for treating first-line advanced RCC in the third quarter of 2017.
Apart from tivozanib, other promising candidates in its pipeline include ficlatuzumab (hepatocyte growth factor inhibitory antibody; phase I – squamous cell carcinoma of the head and neck), AV-203 (ErbB3 (HER3) inhibitory antibody targeting growth differentiating factor-15/GDF15; phase I completed – advanced solid tumors), AV-380 (IgG1inhibitory monoclonal antibody; preclinical – cachexia) and AV-353 (inhibitory antibody specific to Notch 3; preclinical – pulmonary arterial hypertension/PAH).
With no approved products in its portfolio, AVEO Pharma is completely dependent on the success of its pipeline candidates. Any pipeline or regulatory setback will have a negative impact on its share price.
Zacks Rank & Stock to Consider
AVEO Pharma currently carries a Zacks Rank #4 (Sell).
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Zacks' Hidden Trades
While we share many recommendations and ideas with the public, certain moves are hidden from everyone but selected members of our portfolio services. Would you like to peek behind the curtain today and view them?
Starting now, for the next month, I invite you to follow all Zacks' private buys and sells in real time from value to momentum...from stocks under $10 to ETF to option movers...from insider trades to companies that are about to report positive earnings surprises (we've called them with 80%+ accuracy). You can even look inside portfolios so exclusive that they are normally closed to new investors.
Click here for Zacks' secret trade>>
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Bayer AG (DE:BAYGN) (BAYRY): Free Stock Analysis Report
Exelixis, Inc. (EXEL): Free Stock Analysis Report
AVEO Pharmaceuticals, Inc. (AVEO): Free Stock Analysis Report
Original post
Zacks Investment Research