Novo Nordisk (CO:NOVOb) (NYSE:NVO) announced that the FDA has approved its semaglutide once-daily pre-filled pen to improve glycaemic control in type II diabetes patients. The GLP-1 receptor agonist will be marketed by the trade name of Ozempic.
The approval of Ozempic was based on results from the phase III SUSTAIN study. The results showed that people with type II diabetes, who are treated with once-weekly semaglutide experienced statistically significant and sustained blood glucose control compared with Merck’s (NYSE:MRK) Januvia (sitagliptin), Astrazeneca’s (NYSE:AZN) once-weekly Bydureon (exenatide extended-release), Sanofi’s (NYSE:SNY) Lantus (insulin glargine) and placebo. Also, the positive recommendation from an FDA Advisory Committee meeting in October 2017 supported the approval.
Novo Nordisk expects to launch Ozempic in United States in the beginning of 2018. As a part of post-approval requirements, the company will conduct a paediatric trial in patients under 18 years.
Meanwhile, Ozempic is under review by several regulatory agencies including the European Medicines Agency, the Japanese Pharmaceuticals and Medical Devices Agency.
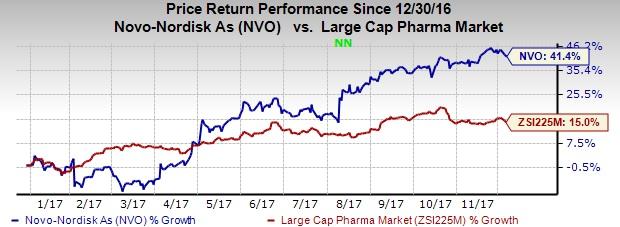
Shares of the company have rallied 41.4% compared with the industry’s gain of 15% on a year-to-date basis.
Additionally, Novo Nordisk is making efforts to develop new treatments for diabetes, which is its core area of expertise. In September 2017, the company received approval for its fast acting insulin aspart, Fiasp. Other than semaglutide, its key pipeline candidate includes nonacog beta pegol. In September 2017, the company received approval for its fast acting insulin aspart, Fiasp.
GLP-1 market, which includes Lilly’s Trulicity and AstraZeneca Plc's once-weekly Bydureon Can give tough competition to Novo Nordisk’s semaglutide.
Zacks Rank
Novo Nordisk carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Wall Street’s Next Amazon (NASDAQ:AMZN)
Zacks EVP Kevin Matras believes this familiar stock has only just begun its climb to become one of the greatest investments of all time. It’s a once-in-a-generation opportunity to invest in pure genius.
Sanofi (PA:SASY) (SNY): Free Stock Analysis Report
Astrazeneca PLC (LON:AZN): Free Stock Analysis Report
Novo Nordisk A/S (NVO): Free Stock Analysis Report
Merck & Company, Inc. (MRK): Free Stock Analysis Report
Original post
Zacks Investment Research
