Johnson & Johnson (NYSE:JNJ) kicked off third quarter earnings season for the pharmaceutical sector on a strong note with the company beating on both earnings and revenues. While focus remained on J&J’s strong earnings results, companies like Allergan (NYSE:AGN) were also in the news regarding patent litigation.
Recap of the Week’s Most Important Stories
J&J Q3 Results Impress, Raises Outlook: J&J’s Q3 earnings results were strong with the company beating on both earnings and revenues. All three segments (Consumer, Medical Devices and Pharmaceuticals) did well with oncology recording robust growth. Results included contribution from pulmonary hypertension products which became a part of the company’s portfolio following its acquisition of Actelion. Based on solid third quarter results, J&J raised its outlook for the year as well (Read more: J&J Beats on Q3 Earnings, Actelion Buyout Drives Sales).
The company has gained 23.3% year to date, compared to the 20.6% rally of the industry it belongs to.
Sanofi’s Dupixent Tops in Allergic Inflammatory Disease Study: Sanofi (PA:SASY) and partner Regeneron Pharmaceuticals announced positive phase II results on Dupixent (dupilumab) in adults with active moderate-to-severe eosinophilic esophagitis, a chronic, allergic inflammatory disease. Patients receiving Dupixent weekly reported a significant improvement in the ability to swallow versus placebo (Read more: Sanofi/Regeneron's Dupixent Succeeds in Phase II Study). The market could be huge for Dupixent if it gains timely approval given the lack of any FDA-approved treatments. Dupixent has orphan drug status in the United States for this indication.
Dupixent, which has blockbuster potential, is currently approved for eczema and is being evaluated for additional indications apart from eosinophilic esophagitis. In fact, last month, the companies had announced positive top-line data from a late-stage study on Dupixent for uncontrolled persistent asthma.
Regeneron and Sanofi are also collaborating with Aimmune Therapeutics, a clinical-stage company focused on the development of treatments for potentially life-threatening food allergies. Under the clinical collaboration, Aimmune’s AR101 will be evaluated with adjunctive Dupixent in peanut-allergic patients in a mid-stage study that is scheduled to commence next year. The study will be sponsored by Regeneron while Aimmune will provide clinical supply of AR101 and food challenge materials.
Data on Merck’s Keytruda, Priority Review for Lynparza sNDA: Merck (NYSE:MRK) presented updated overall survival (“OS”) data on its anti-PD-1 therapy, Keytruda, for the first-line treatment of patients suffering from metastatic non-small cell lung cancer with high levels of PD-L1. The company said that the additional six months of available data continued to show a reduction in the risk of death by 37% for Keytruda compared to chemotherapy. Keytruda more than doubled median OS compared to chemotherapy after two years of follow up.
Keytruda is a key product in Merck’s portfolio with the drug bringing in sales of $1.5 billion in the first half of 2017.
Merck and partner AstraZeneca also announced that they have been granted priority review by the FDA for their supplemental New Drug Application (sNDA) for the use of PARP inhibitor Lynparza tablets in patients with gBRCA, HER2-negative metastatic breast cancer (“MBC”) who have been previously treated with chemotherapy either in the neoadjuvant, adjuvant or metastatic settings. A response from the agency is expected in the first quarter of 2018.
Novo Nordisk (CO:NOVOb) Diabetes Drug Gets FDA Panel Support: Novo Nordisk (NYSE:NVO) got a boost with an FDA advisory panel (the Endocrinologic and Metabolic Drugs Advisory Committee) voting unanimously (16-0) in favor of approving once-weekly semaglutide for use in type II diabetes patients. Given the favorable vote, chances of gaining FDA approval look pretty high. On its second quarter call, Novo Nordisk had said that it expects a decision from the FDA by year end. Semaglutide’s approval would be a major positive for the company with the drug expected to be a key growth driver.
Novo Nordisk is a Zacks Rank #2 (Buy) stock - you can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Priority Review for BMY Drug Label Expansion: Bristol-Myers Squibb Company’s (NYSE:BMY) immuno-oncology therapy, Opdivo, has been granted priority review by the FDA for label expansion into the treatment of melanoma patients who are at high risk of disease recurrence following complete surgical resection. Opdivo, one of the prioritized brands in Bristol-Myers’ portfolio, brought in sales of $2.3 billion in the first half of 2017 (Read more: Bristol-Myers Gets Priority Review for Opdivo Label Expansion).
Adverse Ruling for Allergan in Restasis Patent Litigation: Allergan continues to make headlines related to its blockbuster eye drug, Restasis. This time round, the company announced that it has received an adverse ruling from the U.S. District Court for the Eastern District of Texas regarding four patents covering the drug. The court found the patents to be invalid. Allergan intends to appeal the ruling and said that the FDA is yet to approve any generic version of the drug. However, ophthalmology-focused company, Imprimis Pharmaceuticals, announced that it is working on making lower-cost alternatives to Restasis available. The company said that initial prescriptions will be 99 cents for a one month supply while refills will start at $79 per month, including shipping.
According to Allergan, the Restasis patents are scheduled to expire on Aug 27, 2024. Allergan, which entered into a settlement agreement with a generic challenger recently (InnoPharma), has been adopting different ways to protect Restasis from generic competition. The company came under fire for entering into an agreement with the Saint Regis Mohawk Tribe, with lawmakers questioning the unconventional move adopted by the company to protect the drug from generic competition. Restasis is Allergan’s second best-selling drug bringing in sales of almost $1.5 billion in 2016. Lawmakers pointed out that the sovereign status of Native American tribes would make any patent challenges lengthy and complex as tribes may be immune from the legal claims made by generic drug makers to challenge patents. The deal has basically raised concerns that it will curb generic competition in the pharmaceutical industry and discourage generic drugmakers from making cheaper copy-cat versions of expensive drugs.
Impax to Combine with Amneal: Specialty pharma company Impax Laboratories (NASDAQ:IPXL) will be merging with privately-held generic company Amneal Pharmaceuticals in an all-stock transaction. With this merger, the combined company will have high-value generic product pipelines and a growing specialty business. The deal is expected to boost Impax’s stand-alone earnings per share in the first 12 months and generate double-digit revenue and earnings per share growth over the three years following the close of the transaction. Annual cost synergies of about $200 million are expected to be achieved within three years. The merger is expected to go through in the first half of 2018 (Read more: Impax Laboratories and Amneal Pharmaceuticals Agree to Merge).
Performance
Large Cap Pharmaceuticals Industry 5YR % Return
The NYSE ARCA Pharmaceutical Index was up 1% this week with the sector reacting positively to J&J’s Q3 results. Among major stocks, J&J was up 3.8% while Bristol-Myers declined 1%. Over the last six months, Bristol-Myers was up 23.2% (See the last pharma stock roundup here: Pfizer (NYSE:PFE) Mulls Options for Consumer Healthcare, LLY's Pipeline Setback).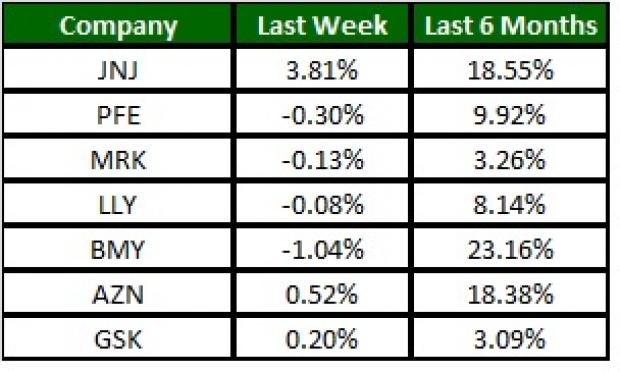
What's Next in the Pharma World?
Watch out for earnings results from companies like Novartis, Eli Lilly (NYSE:LLY) and Bristol-Myers among others in the coming week.
Wall Street’s Next Amazon (NASDAQ:AMZN)
Zacks EVP Kevin Matras believes this familiar stock has only just begun its climb to become one of the greatest investments of all time. It’s a once-in-a-generation opportunity to invest in pure genius.
Click for details >>
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Eli Lilly and Company (LLY): Free Stock Analysis Report
Allergan PLC. (AGN): Free Stock Analysis Report
Novo Nordisk A/S (NVO): Free Stock Analysis Report
Johnson & Johnson (JNJ): Free Stock Analysis Report
Merck & Company, Inc. (MRK): Free Stock Analysis Report
Impax Laboratories, Inc. (IPXL): Free Stock Analysis Report
Original post