Alnylam Pharmaceuticals, Inc. (NASDAQ:ALNY) announced that the FDA has granted Breakthrough Therapy Designation for lead candidate, patisiran. The candidate is an investigational RNAi therapeutic targeting transthyretin (TTR) for the treatment adults with hereditary ATTR (hATTR) amyloidosis with polyneuropathy.
The FDA’s Breakthrough Therapy designation aims at accelerating the development and review of drugs that are intended toward treating serious or life-threatening conditions and provide access to patients as soon as possible.
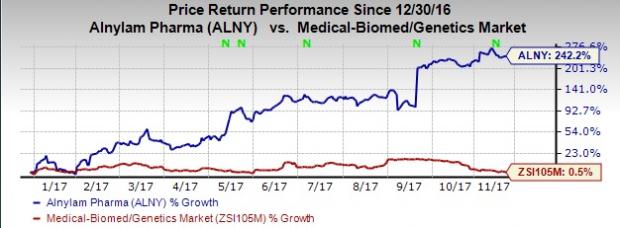
So far this year, shares of Alnylam have skyrocketed 242.2% compared with the industry’s gain of 0.5%.
Last week, the company announced that it has initiated submission of a rolling New Drug Application (NDA) to the FDA for patisiran and expects the last submission by the end of 2017. Also, Alnylam has requested priority review of application for patisiran, if granted, it might result in a six-month review process.
The NDA filing is based on promising results from the APOLLO phase III study that met its primary as well as all secondary endpoints. The patients who were administered patisiran experienced significant improvement in quality of life compared with placebo. The study also demonstrated the first-ever positive results for an RNAi therapeutic.
Recently, Alnylam announced that the Committee for Medicinal Products for Human Use (“CHMP”) of the European Medicines Agency (“EMA”) has granted an accelerated assessment for patisiran. With the accelerated assessment, the company can expect the review timeline to be reduced from the typical 210 days to 150 once the marketing authorization application (MAA) is filed and validated in the EU.
Furthermore, Alnylam and its partner Sanofi (NYSE:SNY) plans to submit Marketing Authorisation Application to the European Medicines Agency around the year-end.
Meanwhile, Sanofi is preparing to start regulatory filings for patisiran in Japan, Brazil and other countries in the first half of 2018. Once it gets the required regulatory approvals, Alnylam will commercialize patisiran in the United States, Canada and Western Europe with Sanofi commercializing the product in the rest of the world.
Going forward, the potential approval of patisiran is likely to be a huge boost and will be an important treatment option for the people suffering from this often fatal disease.
Zacks Rank & Stocks to Consider
Alnylam carries a Zacks Rank #3 (Hold). Some better-ranked health care stocks in the same space include Sucampo Pharmaceuticals, Inc. (NASDAQ:SCMP) andLigand Pharmaceuticals Incorporated (NASDAQ:LGND) . While Sucampo sports a Zacks Rank#1 (Strong Buy), Ligand holds a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Sucampo’searnings per share estimates have moved up from $1.01 to $1.12 for 2017 and from $1.06 to $1.19 for 2018 over the last 30 days. The company delivered positive earnings surprises in three of the trailing four quarters, with an average beat of 15.63%.
Ligand’s earnings per share estimates have climbed $3.68 to $3.70 for 2018 over the last 60 days. The company pulled off positive earnings surprises in two of the trailing four quarters, with an average beat of 8.22%. The share price of the company has increased 28.4% year to date.
Zacks' Hidden Trades
While we share many recommendations and ideas with the public, certain moves are hidden from everyone but selected members of our portfolio services. Would you like to peek behind the curtain today and view them?
Starting now, for the next month, I invite you to follow all Zacks' private buys and sells in real time from value to momentum...from stocks under $10 to ETF to option movers...from insider trades to companies that are about to report positive earnings surprises (we've called them with 80%+ accuracy). You can even look inside portfolios so exclusive that they are normally closed to new investors.
Click here for Zacks' secret trade>>
Sanofi (SNY): Free Stock Analysis Report
Alnylam Pharmaceuticals, Inc. (ALNY): Free Stock Analysis Report
Ligand Pharmaceuticals Incorporated (LGND): Free Stock Analysis Report
Sucampo Pharmaceuticals, Inc. (SCMP): Free Stock Analysis Report
Original post
Zacks Investment Research
