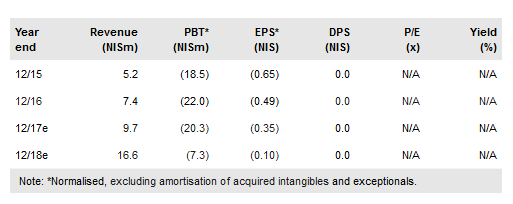
Gardia Medical’s Wirion device is on track for FDA submission, expected by end-2017; approval is possible in Q218. If approved, it would become the only embolic protection system for all atherectomy procedures in the legs which we think will help to reach a strategic transaction. Furthermore, Allium Medical Solutions Ltd (TA:ALMD) Stents and IBI Medical are expected to gain approval in Russia in 2018 while approval in China is expected in early 2018. We expect initial revenues from Mexico in Q417 and from the strategic agreement in Russia in H118. In addition, Allevetix is due to start a clinical trial in the next few months and TruLeaf is progressing its large animals study. Our updated valuation is NIS1.68/share.
Gardia’s Wirion system nears FDA submission
Gardia is pursuing US approval of its Wirion device following strong data from the WISE-LE trial which met its primary and secondary endpoints early. Positive data on debris capture has been released and will be presented at the ISET conference in Florida in February 2018. A 510(k) submission to the FDA is expected by the end of the year; an approval decision could come in Q218. If approved, Wirion would become the only protection system cleared for all atherectomy procedures in the US. The company is advancing towards a strategic transaction for Gardia. We model Wirion revenue of NIS2.8m after full launch in 2018e, to NIS8.6m in 2020e.
To read the entire report Please click on the pdf File Below: