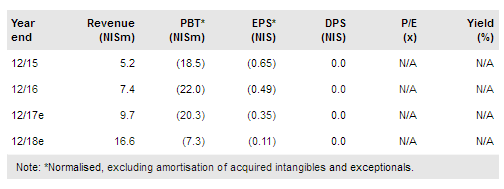
Allium Medical Solutions Ltd (TA:ALMD) has announced that Gardia Medical’s Wirion device has met the primary endpoint of its clinical trial, an important milestone for strategic partnering discussions. In other news, on 13 August Allium reported H117 financial results, with revenues down 5.7% y-o-y to NIS3.7m. The company has also announced approval of its prostatic and urethral stents in Mexico and expects to launch sales in H217. However, registration of Allium Stents and IBI Medical in Russia is taking longer than expected due to a lengthier and more complex regulatory process. We note that regional expansion is crucial for the company’s investment case in the long run. Updated for the recent newsflow, our valuation of Allium is now NIS1.89/share.
Gardia’s clinical trial positive at interim analysis
After it was reported that the WISE-LE trial met the endpoint at an early stage, the Wirion device is on track to become the only protection system cleared for all atherectomy procedures in the US, including the rapidly growing large lower extremities indication, if approved. The company is in partnering discussions and we believe the chances of striking a deal have increased. We model NIS2.8m revenue on full launch of Wirion in 2018e, growing to NIS8.6m in 2020e.
To read the entire report Please click on the pdf File Below: