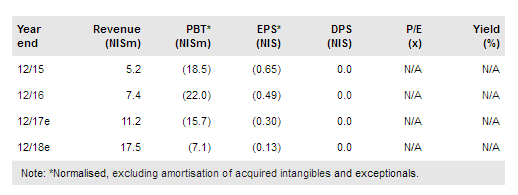
Allium Medical (TA:ALMD) has made significant progress across business lines. The safety monitoring board has recommended continuing the study of Gardia’s embolic protection device; clinical data will be reported by end Q317. A clinical trial of its urological stents in the local population will not be necessary for approval in China; this is in line with our expectations. We expect sales to start in 2018 via a NIS58m eight-year distribution agreement. Following positive preclinical data, Allevetix is on track to start a clinical trial by year-end 2017. We forecast revenue CAGR of 41% in 2016-20e and expect the company to break even in 2020. Our valuation is NIS1.95-2.08/share.
Continue trial after positive safety assessment
A Data Safety Monitoring Board (DSMB) has recommended continuation of the study of the Wirion embolic prevention device; clinical data will be reported by end Q317. If data are positive, and FDA approves the product, Wirion will become the only protection system cleared for all atherectomy procedures in the US; at that point, we believe chances to strike a partnership will increase. There is strong interest in the market for atherectomy procedures, as demonstrated by the recent acquisition of Spectranetics Corporation by Philips for a total of €1.9bn.
To read the entire report Please click on the pdf File Below: