Alexion Pharmaceuticals, Inc. (NASDAQ:ALXN) announced results from an interim analysis of an ongoing phase III REGAIN study of lead drug Soliris for the treatment of patients with refractory generalized myasthenia gravis (gMG), who are anti-acetylcholine receptor (AChR) antibody-positive.
We note that, Soliris is approved for the treatment of two severe and ultra-rare disorders resulting from chronic uncontrolled activation of the complement component of the immune system, paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS).
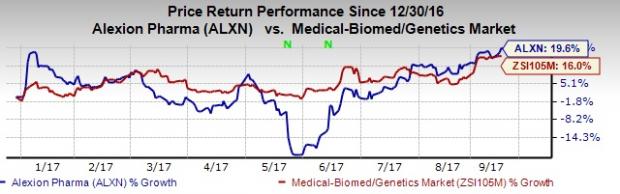
Alexion’s shares have outperformed the industry year to date. The stock has been up 19.6% compared with the industry’s gain of 16% in the same time frame.
Coming back to the release, the study showed sustained treatment benefits of Soliris treatment for patients with refractory generalized myasthenia gravis. Additionally, it revealed that safety profile of Soliris was consistent with that observed in the REGAIN study.
Furthermore, the evaluation showed that the benefits for patients treated with Soliris in REGAIN through 26 weeks were maintained in the extension study across all four assessment scales for an additional 52 weeks (78 weeks in total). In fact, the patients who received placebo in REGAIN and then were treated with Soliris in the extension study, demonstrated significant treatment benefits within 1 to 4 weeks and were sustained through 52 weeks across all four assessment scales.
Meanwhile, Alexion has filed regulatory applications with the FDA as well as the European Medicines Agency (EMA) for Soliris for the treatment of refractory gMG in patients who are anti-acetylcholine receptor antibody-positive. The FDA accepted the supplemental Biologics License Application (sBLA) and set a Prescription Drug User Fee Act date of Oct 23.
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has also adopted a positive opinion for the same and the final decision from the European Commission (EC) is anticipated in the third quarter of 2017.
Additionally, a phase III study (PREVENT) on Soliris in patients with relapsing neuromyelitis optica spectrum disorder is ongoing with enrollment being expected to be completed in 2017 and data expected in 2018. Label expansion into additional indications would give Soliris access to a higher patient population and increase the commercial potential of the drug significantly.
Zacks Rank & Key Picks
Alexion currently sports a Zacks Rank #1 (Strong Buy). Some other top-ranked stocks in health care sector include Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN) , ACADIA Pharmaceuticals Inc. (NASDAQ:ACAD) and Aduro BioTech, Inc. (NASDAQ:ADRO) . While Regeneron carries the same bullish rank as Alexion, ACADIA and Aduro hold a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
ACADIA Pharmaceuticals loss estimates per share have narrowed from $2.59 to $2.57 for 2017 and from $1.92 to $1.90 for 2018 over the last 30 days. The company pulled off positive earnings surprises in two of the trailing four quarters, with an average beat of 7.97%. The share price of the company has increased 25.7% year to date.
Regeneron’s earnings per share estimates have increased from $12.83 to $14.99 for 2017 and from $15.38 to $16.64 for 2018 over the last 30 days. The company delivered positive earnings surprises in two of the trailing four quarters, with an average beat of 10.11%. The share price of the company has increased 17.6% year to date.
Aduro’s loss estimates per share have narrowed from $1.46 to $1.32 for 2017 and from $1.55 to $1.24 for 2018 over last 30 days. The company came up with positive earnings surprises in two of the trailing four quarters, with an average beat of 2.53%.
5 Trades Could Profit "Big-League" from Trump Policies
If the stocks above spark your interest, wait until you look into companies primed to make substantial gains from Washington's changing course.
Today Zacks reveals 5 tickers that could benefit from new trends like streamlined drug approvals, tariffs, lower taxes, higher interest rates, and spending surges in defense and infrastructure.
See these buy recommendations now >>
Alexion Pharmaceuticals, Inc. (ALXN): Free Stock Analysis Report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Aduro Biotech, Inc. (ADRO): Free Stock Analysis Report
ACADIA Pharmaceuticals Inc. (ACAD): Free Stock Analysis Report
Original post
Zacks Investment Research