Agios Pharmaceuticals (NASDAQ:AGIO)
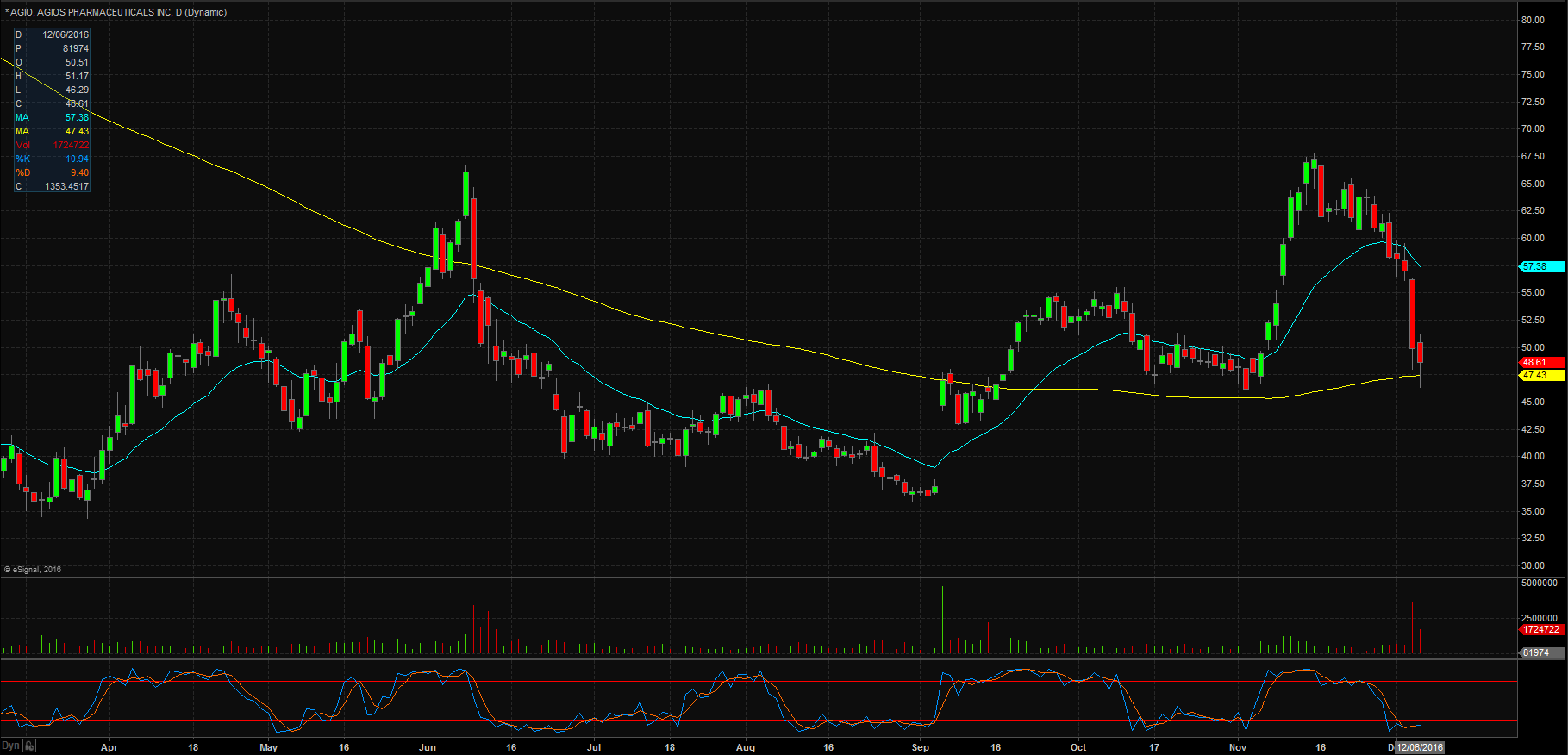
Over the weekend, Agios Pharmaeucticals released safety data from one of its clinical trials for AG-348 and AG-519 that showed adverse effects on its patients. The news was released at the American Society of Hematology’s annual meeting during the weekend and when the markets opened on Monday shares of AGIO were beat down over 10%. As you can see in the daily chart below, shares have run into their daily 200-day moving average where they have found a little bit of support. We will want to see prices hold that level otherwise we could be in for another leg down.
What is interesting is that AGIO also released positive data on one of its trials involving AG-120, in which it demonstrated improvements in patients. With mixed news like this, we will want to see shares stable off before making any trade decisions.
Agios Pharmaceuticals Notes
Courtney DiNardo, M.D., lead investigator and assistant professor, department of leukemia at the University of Texas MD Anderson Cancer Center said,
AG-120 continues to demonstrate an impressive single-agent efficacy and safety profile in this cohort of high-risk relapsed or refractory AML patients, with some responses maintained for approximately two years...In addition, new molecular data for AG-120 suggests some patients experience clearance of the IDH1 mutant gene in their blood or bone marrow as assessed by next generation sequencing, demonstrating the depth of response that can occur with AG-120 therapy.
AGIO Profile
Agios Pharmaceuticals, Inc. is a biopharmaceutical company which engages in applying scientific leadership in the field of cellular metabolism to transform the lives of patients with cancer and rare genetic disorders of metabolism. Its products include IDH1 AND IDH2, PKR, II D-2 hydroxyglutaric aciduria, AG-221 and AG-120. The comany was founded by Lewis Clayton Cantley, Tak W. Mak, Craig B. Thompson and Shin-Shan Michael Su on August 7, 2007 and is headquartered in Cambridge, MA.