Agios Pharmaceuticals, Inc. (NASDAQ:AGIO) announced that it has submitted a new drug application (“NDA”) to the FDA for its investigational candidate ivosidenib (AG-120) for treatment of patients with relapsed or refractory acute myeloid leukemia (R/R AML) and an isocitrate dehydrogenase-1 (IDH1) mutation.
Notably, the company has requested the regulatory agency for a priority review keeping in mind that the latter would have to complete the process within a span of six months if it accepts the application.
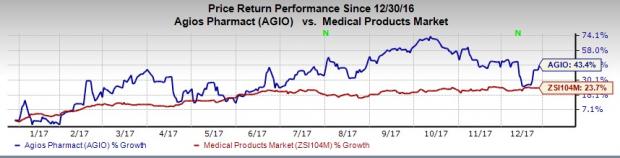
Agios’ shares increased almost 3% on Dec 26, following the news release. Moreover, the stock has outperformed the industry so far this year. It has surged 43.4% compared with the industry’s rally of 23.7%.
The NDA submission of was based on encouraging data from dose expansion part of an ongoing phase I study, evaluating ivosidenib, as a single agent on patients with advanced hematologic malignancies and an IDH1 mutation. The phase I trial is assessing the safety and tolerability of ivosidenib on advanced solid tumors including glioma, intrahepatic cholangiocarcinoma and chondrosarcomas. Earlier this month, the company presented data from the study at the annual meeting of American Society of Hematology in Atlanta.
Ivosidenib is also being evaluated in a phase III study to examine ivosidenib in front-line AML patients with an IDH1 mutant-positive advanced cholangiocarcinoma.
We remind investors that in May 2016, Agios announced an agreement with Celgene Corporation (NASDAQ:CELG) in the field of metabolic immuno-oncology. The companies also agreed to amend certain rights under their 2010 collaboration with Agios gaining full global development and commercialization rights to ivosidenib. Previously, Agios had only U.S. rights to ivosidenib.
Agios is conducting a phase I/II program in combination with Celgene's Vidaza for treating newly diagnosed AML patients, not eligible for intensive chemotherapy. The FDA granted an orphan drug designation to ivosidenib for treatment of AML patients.
In the same press release, the company also announced that the FDA has accepted its investigational new drug application for AG-270 to proceed with phase I dose-escalation study to evaluate the candidate for treatment of multiple tumor types carrying an MTAP deletion. The company expects to initiate the phase I study in the first half of 2018.
Zacks Rank & Key Picks
Agios carries a Zacks Rank #3 (Hold). Two better-ranked stocks in the health care sector are Corcept Therapeutics Incorporated (NASDAQ:CORT) and Achillion Pharmaceuticals, Inc. (NASDAQ:ACHN) , both carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Corcept’s earnings per share estimates have been revised upward from 78 cents to 88 cents for 2018 over the last 60 days. The company delivered positive earnings surprises in two of the trailing four quarters with an average beat of 14.32%. Share price of the company has skyrocketed 158.8% year to date.
Achillion has seen the Zacks Consensus Estimate of a loss per share for 2017 being narrowed from 65 cents to 63 cents and from 74 cents to 67 cents for 2018 over the last 60 days. The company came up with positive earnings surprises in two of the last four quarters with an average beat of 4.51%.
Wall Street’s Next Amazon (NASDAQ:AMZN)
Zacks EVP Kevin Matras believes this familiar stock has only just begun its climb to become one of the greatest investments of all time. It’s a once-in-a-generation opportunity to invest in pure genius.
Agios Pharmaceuticals, Inc. (AGIO): Free Stock Analysis Report
Celgene Corporation (CELG): Free Stock Analysis Report
Achillion Pharmaceuticals, Inc. (ACHN): Free Stock Analysis Report
Corcept Therapeutics Incorporated (CORT): Free Stock Analysis Report
Original post
Zacks Investment Research