Positive preclinical data for dipraglurant in dystonia supports progression into a Phase II study for rare dystonias in H113. Phase II results (Q413), if positive, could trigger a larger Phase IIb trial (2014). Addex (ADXN.SIX) continues to target a dipraglurant partnership for Parkinson’s disease (PD), which may be informed by upcoming Phase II results (Q213) for Novartis’s competing mGlu5 inhibitor (AFQ056). While Addex is currently financed to end-2013, potential deals and/or financings could extend the cash runway. A planned NASDAQ listing (timing tbc) in our view, could be a prelude to a financing.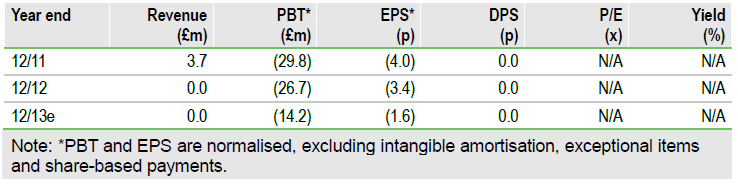
Positive dipraglurant preclinical data in dystonia…
Addex has reported positive preclinical data for dipraglurant (an oral mGlu5 inhibitor) in a validated model of primary generalised tortional dystonia 1 (DYT1), a severe genetic form of dystonia (involuntary muscle spasms). The drug showed a dose-dependent normalisation of neuronal over-excitation in the brains of transgenic DYT1 mice. These results build on the existing body of supportive preclinical data (tottering mouse model, MPTP monkey) for dipraglurant in treating rare dystonia.
…supports progression into Phase II trial in H113
Positive preclinical data for dipraglurant in dystonia are supported by anecdotal findings in the Phase II study in Parkinson’s disease levodopa-induced dyskinesia (PD-LID), where the drug reduced L-dopa-induced dystonia in four patients. We expect a pilot Phase II study in rare dystonia to start in Q213 and render data in Q413. In parallel, Addex continues to target a partnership for the PD indication. Separately, Novartis’s Phase II study of the modified dose formulation of AFQ056 (oral mGlu5 inhibitor) in PD-LID is due to readout shortly (April 2013); this could determine the fate of this compound (a potential positive/negative catalyst for Addex), which has shown activity in PD-LID but with tolerability issues.
Financials: Cash runway into late 2013
Addex had cash of CHF15.3m at year-end 2012. We project operating expenditure of CH15m for 2013, including restructuring related costs. On these projections, and barring new partnerships and/or financing, Addex is financed to year-end 2013.
Valuation: Risk-adjusted NPV of CHF218m
We value Addex at CHF218m ($234m) or CHF25.40 per share. Our rNPV assumes industry-standard success rates for drugs based on their development stage and a hypothetical 18% royalty on dipraglurant and 12% on JNJ-40411813.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Addex Therapeutics: Cash Runway Into Late 2013
Published 04/19/2013, 06:21 AM
Updated 07/09/2023, 06:31 AM
Addex Therapeutics: Cash Runway Into Late 2013
Dipraglurant data supports orphan disease focus
Latest comments
Loading next article…
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2025 - Fusion Media Limited. All Rights Reserved.