Adamas Pharma (NASDAQ:ADMS)
Adamas Pharmaceuticals, Inc. (ADMS), a pharmaceutical company yesterday announced that the Food and Drug Administration (FDA) has approved GOCOVRI. GOCOVRI is the company’s drug treatment of dyskinesia in patients with Parkinson’s disease.
GOCOVRI is used to treat the abnormal or involuntary movements associated with Parkinson’s, the only drug approved by the FDA for this purpose. Adamas plans to release the drug to the public by the fourth quarter with full deployment by January 2018.
Adamas Pharmaceuticals, Inc. CEO’s Comments
“Today’s approval is a tremendous milestone for Adamas and for the Parkinson’s disease community,” said Gregory T. Went, Ph.D., Founder, Chairman and Chief Executive Officer of Adamas Pharmaceuticals, Inc. “GOCOVRI has the potential to help people with Parkinson’s disease suffering from dyskinesia by finally providing physicians with an effective tool to address this long-standing unmet medical need. We thank the physicians, clinical staff, patients and their families who participated in the clinical trials for making this advancement possible for the community.” Globe Newswire
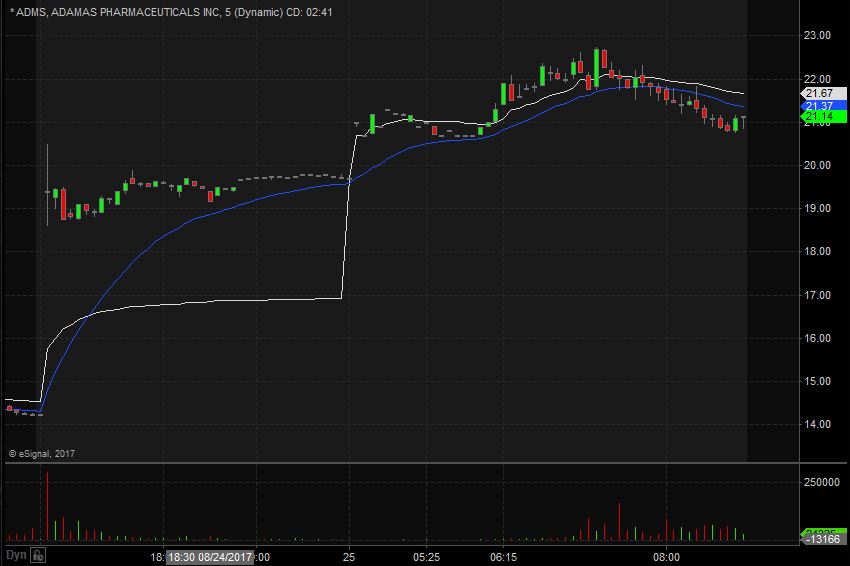
ADMS Technical Analysis
ADMS opened trading yesterday at $15.29 which was up from the previous day’s trading close of $14.23. ADMS closed trading yesterday at $14.23 and spiked up in the after hours to $19.20, equivalent to a 35% increase from the closing price. Taking a look at the daily chart we can see the last time ADMS traded above these levels we have to go back to January 6th when it traded at highs of $19.50.
Taking a closer look at the daily chart we can see that before the spike up ADMS had been in an overall downward trend dating back to July 24th when it traded at $18.45. ADMS has a float of 11.16 million shares and traded 3.94 times the normal daily trading volume on Thursday.
For trading purposes, I would like to see ADMS open trading on Friday above $17.50 and if it does I would be looking to take a long position at the bell. My stop loss would be $0.30 from my entry position fearing anything more than that and the stock would start to fill in the gap up.
Company Profile
Adamas Pharmaceuticals, Inc., a pharmaceutical company, discovers, develops, and sells chrono-synchronous therapies for chronic neurologic disorders. The company’s product portfolio comprises ADS-5102, a chrono-synchronous amantadine therapy for the treatment of levodopa-induced dyskinesia in patients with Parkinson’s disease.
Its portfolio also comprises of Namzaric (memantine hydrochloride extended-release and donepezil hydrochloride) capsules; and Namenda XR (memantine hydrochloride) extended release capsules for the treatment of moderate to severe Alzheimer’s disease. The company’s products under development include ADS-4101, a chrono-synchronous lacosamide therapy that has completed first Phase I clinical study for the treatment of partial onset seizures in patients with epilepsy.
The company was formerly known as NeuroMolecular Pharmaceuticals, Inc. and changed its name to Adamas Pharmaceuticals, Inc. in July 2007. Adamas Pharmaceuticals, Inc. was founded in 2000 and is headquartered in Emeryville, California.