AbbVie, Inc. (NYSE:ABBV) announced that the FDA has lifted the partial clinical hold on the phase III study — CANOVA — evaluating Venclexta (venetoclax) in patients with relapsed/refractory multiple myeloma. The study is comparing a combination of Venclexta and dexamethasone with Celgene’s (NASDAQ:CELG) Pomalyst (pomalidomide) plus dexamethasone in patients with translocation (11;14) abnormality, the most common genetic abnormalities in patients with multiple myeloma.
The FDA’s decision followed the company’s agreement to revise the CANOVA study protocol, which includes new risk mitigation measures, protocol-specified guidelines and updated futility criteria. The company will re-initiate enrollment in the study at sites where the protocol has been approved.
Please note that in March, the FDA had placed all studies evaluating Venclexta for the treatment of multiple myeloma on partial clinical hold. Currently, all other studies, except for CANOVA study, are under partial clinical hold.
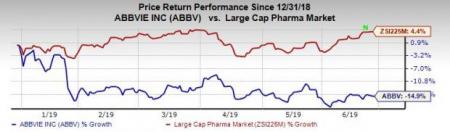
Shares of AbbVie have decreased 14.9% so far this year against the industry’s rise of 4.4%.
The FDA had placed the partial clinical hold following the higher proportion of deaths observed in the Venclexta arm compared to the control arm of the phase III BELLINI study.
The BELLINI study evaluated the safety and efficacy of Venclexta plus Takeda’s (NYSE:TAK) Velcade (bortezomib) and dexamethasone in patients with relapsed or refractory multiple myeloma who are considered sensitive or naïve to proteasome inhibitors and have received one to three prior lines of therapy. The combination was being compared to treatment with Velcade, dexamethasone and placebo.
Venclexta is presently approved for other cancer types like newly-diagnose chronic lymphocytic leukemia (“CLL”) and second-line treatment of certain acute myeloid leukemia (“AML”) patients. The clinical hold does not impact any of the approved indications for Venclexta. It is presently not approved to treat multiple myeloma.
Venclexta is jointly marketed by AbbVie and Roche’s (OTC:RHHBY) pharma arm, Genentech in the United States and by AbbVie outside the United States. Venclexta is marketed by the trade name of Venclyxto in the EU.
For AbbVie, Venclexta brought in revenues of $151 million in the first quarter of 2019, up more than 150% year over year driven by uptake in the second-line plus setting following approval in the United States and EU in the broad relapsed/refractory CLL segment (MURANO study) in 2018. Venclexta is a key drug in AbbVie’s oncology portfolio. The drug had generated sales of $344 million in 2018.
AbbVie is studying Venclyxto/Venclexta to expand the label to address the broader relapsed/refractory CLL patient population, expand into earlier lines of therapy and broaden into other hematologic malignancies like multiple myeloma and AML. However, pipeline setbacks like the clinical hold on multiple myeloma studies will hurt growth prospects of this important oncology drug.
Zacks Rank
AbbVie currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Looking for Stocks with Skyrocketing Upside?
Zacks has just released a Special Report on the booming investment opportunities of legal marijuana.
Ignited by new referendums and legislation, this industry is expected to blast from an already robust $6.7 billion to $20.2 billion in 2021. Early investors stand to make a killing, but you have to be ready to act and know just where to look.
See the pot trades we're targeting>>
AbbVie Inc. (ABBV): Free Stock Analysis Report
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Celgene Corporation (CELG): Free Stock Analysis Report
Takeda Pharmaceutical Co. (TAK): Free Stock Analysis Report
Original post
Zacks Investment Research