Johnson & Johnson’s (NYSE:JNJ) subsidiary Janssen announced top-line results from the phase III DISCOVER 1 and 2 studies on its IL-23 inhibitor, Tremfya/guselkumab. Both studies evaluated the safety and efficacy of Tremfya as compared to placebo for treating adult patients with active moderate to severe psoriatic arthritis (PsA).
The studies met the primary endpoint of American College of Rheumatology 20% improvement while the safety profile was consistent with the previous programs on Tremfya/guselkumab.
Tremfya was first approved in the United States and the EU in 2017 for the treatment of moderate-to-severe plaque psoriasis.
Based on data from the two DISCOVER studies, Janssen will submit a regulatory filing to the FDA and the European Medicines Agency (EMA) for an approval to expand the label of Tremfya as a treatment for psoriatic arthritis, later this year.
The company plans to present data from the DISCOVER program at an upcoming medical conference.
The double-blind, phase III DISCOVER-1 and DISCOVER-2 studies evaluated the safety and the efficacy of Tremfya (subcutaneous) in patients with active PsA as compared to the placebo arm. Apart from the primary endpoint, the program also assessed several secondary endpoints. The DISCOVER-2 probe analyzed the effect on structural damage (vdH-S) as a key secondary endpoint.
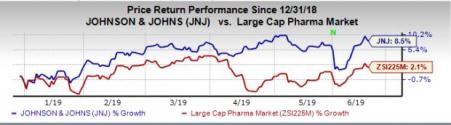
Shares of J&J have increased 8.5% so far this year, outperforming the industry’s rise of 2.1%.
Notably, Tremfya is off to a promising start following its launch in 2017 and has contributed significantly to the company’s growth ever since. The drug recorded sales of $217 million in the first quarter of 2019 compared with $175 million in fourth-quarter 2018.
Meanwhile, Tremfya proved its superiority over the other drugs in the past. Last December, J&J announced results from a head-to-head phase III ECLIPSE evaluation, which compared Tremfya with Novartis' (NYSE:NVS) psoriasis drug, Cosentyx (secukinumab).
The outcomes showed that after a 48-week treatment, 84.5% of the patients treated with Tremfya achieved 90% improvement in disease symptoms as measured by the Psoriasis Area Severity Index in comparison to 70% with respect to Novartis's Cosentyx.
The drug is also being studied for various other indications as well. A phase IIb study is evaluating Tremfya for the treatment of Crohn’s disease. Two other phase II studies are investigating the drug for ulcerative colitis and hidradenitis suppurativa.
Zacks Rank & Stocks to Consider
J&J currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the large cap pharma sector include AbbVie Inc. (NYSE:ABBV) and Merck & Co., Inc. (NYSE:MRK) , both sporting a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
AbbVie’ earnings estimates have been revised 1.5% upward for 2018 and 1.4% for 2019 over the past 60 days.
Merck’s earnings estimates have moved 1.7% north for 2019 and 0.6% for 2020 over the past 60 days. The stock has rallied 8.1% year to date.
Radical New Technology Creates $12.3 Trillion Opportunity
Imagine buying Microsoft (NASDAQ:MSFT) stock in the early days of personal computers… or Motorola (NYSE:MSI) after it released the world’s first cell phone. These technologies changed our lives and created massive profits for investors.
Today, we’re on the brink of the next quantum leap in technology. 7 innovative companies are leading this “4th Industrial Revolution” - and early investors stand to earn the biggest profits.
See the 7 breakthrough stocks now>>
Merck & Co., Inc. (MRK): Free Stock Analysis Report
Novartis AG (NVS): Free Stock Analysis Report
AbbVie Inc. (ABBV): Free Stock Analysis Report
Johnson & Johnson (JNJ): Free Stock Analysis Report
Original post
Zacks Investment Research
