ACADIA Pharmaceuticals Inc (NASDAQ:ACAD)
On Tuesday before the market opened, ACADIA Pharmaceuticals announced that they reached positive top-line results in its Phase II study of pimavanserin in patients with Alzheimer’s disease psychosis. The study met the primary endpoint with statistical significance and showed that patients tolerated the treatment within the safety parameters.
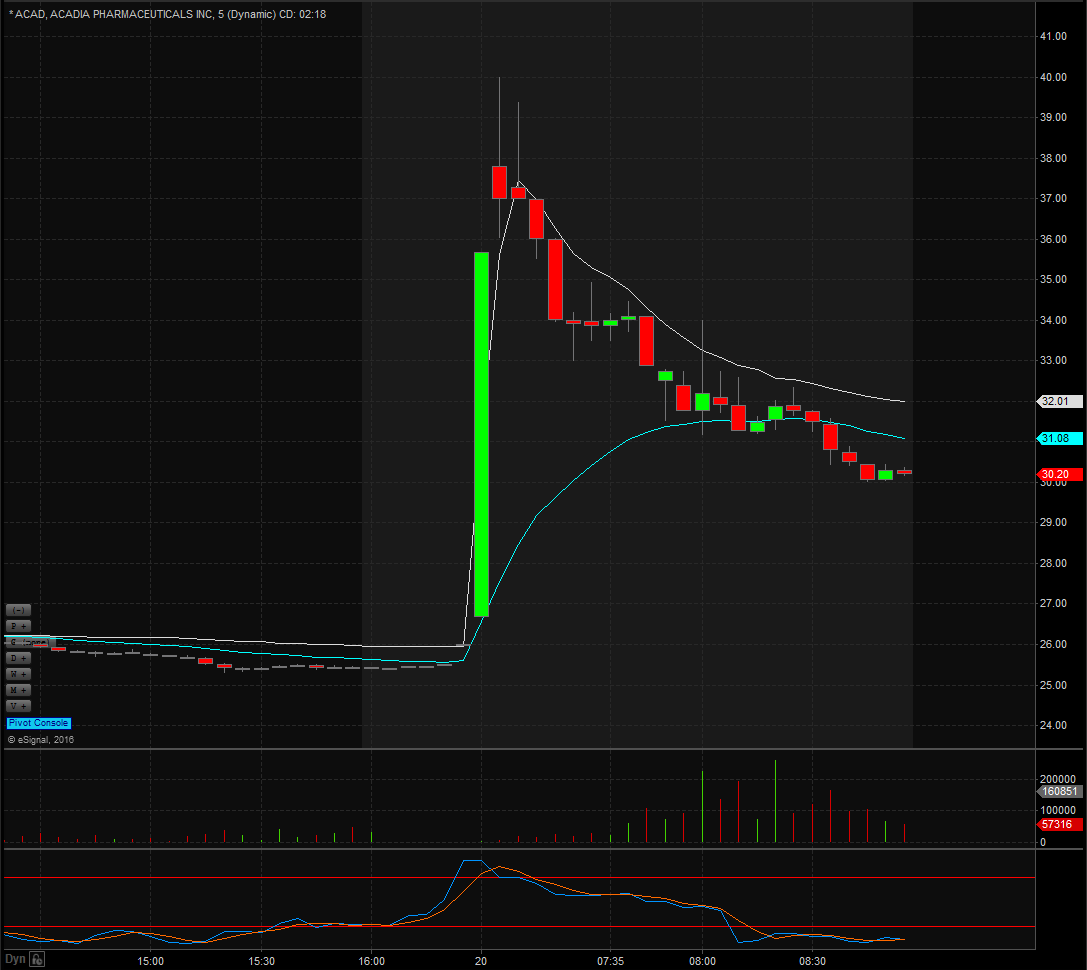
ACAD Technicals
Shares of ACAD spiked to highs of $40 in the pre-market session after closing the previous day at $25.43 marking a 57% pop in price. Shares came off highs and opened the day at $29.26 and should see some resistance at the 200-day moving average currently sitting at $30.59. Look for support to come in at the 20-day moving average currently at $26.66 and $26.50 which has been an important pivot spot on the daily chart. Look for shares to get back above the 200-day moving average to show that bulls have taken over and until it does be careful of going long.
CEO Comments
“Alzheimer’s disease patients suffer from a number of debilitating symptoms, of which psychosis carries a poor prognosis and is associated with earlier placement into nursing homes,” said Steve Davis, ACADIA’s President and Chief Executive Officer. “Data from the -019 Study provide solid evidence that pimavanserin can improve psychosis in another major neurological disorder and provide strategic momentum for the further development of pimavanserin to address the needs of AD Psychosis patients.” – Businesswire
Company Profile
ACADIA Pharmaceuticals Inc. is a biopharmaceutical company. The Company is focused on the development and commercialization of medicines for central nervous system disorders. The Company’s lead drug candidate, NUPLAZID (pimavanserin), is under development for the treatment of Parkinson’s disease psychosis (PDP). NUPLAZID is a selective serotonin inverse agonist (SSIA), targeting 5-HT2A receptors. The Company’s Pimavanserin is a chemical entity, which has completed Phase III development, and is indicated for the treatment of Parkinson’s disease psychosis. NUPLAZID (pimavanserin) is a selective serotonin inverse agonist preferentially targeting the 5-HT2A receptor, a key serotonin receptor that plays a role in psychosis. Through this mechanism, NUPLAZID has demonstrated efficacy in Parkinson’s disease psychosis in its Phase III pivotal trial and avoids the side effects of existing antipsychotics.