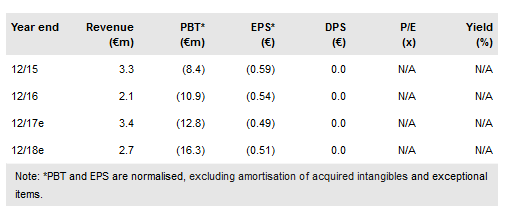
Over the past several months 4SC has reported progress with both its clinical-stage assets – resminostat and 4SC-202. The pivotal trial with resminostat as a maintenance therapy in advanced CTCL passed the first DSMB review and is on track to report data in H119. The Phase Ib/II study with 4SC-202 for melanoma has been initiated, while another Phase II study with 4SC-202 for GI cancer should start in Q118. 4SC-208 completes the core portfolio and is expected to enter the clinic in early 2019. Our valuation is largely unchanged at €347m or €11.3/share (€344m previously).
Pivotal RESMAIN: First DSMB review passed
On 31 January 2018, 4SC reported that an independent data safety monitoring board (DSMB) had recommended the continuation of the ongoing pivotal RESMAIN study with resminostat, a pan-histone deacetylase (HDAC) inhibitor. 4SC remains blinded to the data and efficacy analysis was not part of the review, therefore the continuation without any changes to the protocol was the best possible outcome. Two additional DSMB reviews are planned until the study is fully enrolled with 150 patients. As a reminder, the trial is designed to uniquely position resminostat as a maintenance therapy to make remissions more durable for patients with advanced cutaneous T-cell lymphoma (CTCL) who have achieved remission with systemic therapy (detailed analysis in our last outlook report). 4SC reiterated that the study is on track for the data read-out in H119.
To read the entire report Please click on the pdf File Below: