
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Hutchison China Meditech - WCLC: Positive Data Highlights Nsclc Pipeline

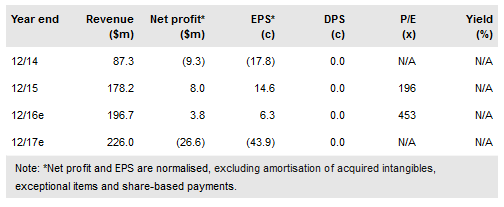
At the recent IASLC World Conference on Lung Cancer, Hutchison China MediTech Ltd (NASDAQ:HCM) published positive proof of concept data in NSCLC on key assets fruquintinib and epitinib. Affirmative data not only translates to further pipeline progression but critically underpins the R&D effort at HCM, fuelling the prospects of a fruquintinib regulatory filing in China in 2018. In 2017 we anticipate the initiation of three more pivotal trials, further data from the mid- to late-stage pipeline, including savolitinib data (papillary renal cell carcinoma, PRCC, and non-small cell lung cancer, NSCLC), and importantly potential China/US NDA submissions. We value HCM at $2.4bn or £32.2 share.
Spotlight on NSCLC: New data at IASLC WCLC
The recent proof of concept data published at IASLC WCLC in both the Phase II NSCLC trial with fruquintinib and the Phase Ib NSCLC trial with epitinib is highly encouraging. Importantly HCM has three TKIs in clinical development for NSCLC; each drug has a specific positioning and is truly differentiated: savolitinib (C-Met), fruquintinib (VEGFR) and epitinib (EGFR). Savolitinib has potential to be HCM’s first internationally launched asset (expect launch in US/EU/Japan in 2018). Depending on the strength of the NDA submission packages and speed of China FDA, we anticipate fruquintinib launch in China in 2018 and epitinib in 2019/20.
To read the entire report Please click on the pdf File Below
Related Articles

Since the Robotaxi event on October 11th, Tesla (NASDAQ:TSLA) stock is up 38%, currently priced at $291.60 per share This is a return to the early November 2024 price level. But...

The Q4 2024 earnings season tapers off from here, with S&P 500® EPS growth surpassing 17%, the highest in 3 years Large cap outlier earnings dates this week include:...

Shares of Alibaba (NYSE:BABA) are on a tear to start off 2025. The consumer discretionary and tech stock is up by 52% this year as of the Feb. 25 close. The company’s cloud...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.