
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Roche Gets FDA Approval For Actemra Trials In COVID-19

Roche (OTC:RHHBY) announced that the FDA has approved a randomized, double-blind, placebo-controlled phase III study to evaluate the safety and efficacy of intravenous rheumatoid arthritis (RA) drug, Actemra (tocilizumab), plus standard of care in hospitalized adult patients with severe COVID-19 pneumonia.
The study will be conducted in collaboration with the Biomedical Advanced Research and Development Authority (BARDA).
The primary and secondary endpoints include clinical status, mortality, mechanical ventilation and intensive care unit (ICU) variables. Patients will be followed for 60 days, post-randomization, and an interim analysis will be conducted to look for early evidence of efficacy.
Moreover, Genentech will provide 10,000 vials of Actemra to the U.S. Strategic National Stockpile for potential future use at the direction of the U.S. Department of Health and Human Services (HHS).
Earlier, Roche received an FDA Emergency Use Authorisation for the cobas SARS-CoV-2 Test to detect the novel virus that causes the COVID-19 disease.
Several independent clinical studies are ongoing worldwide to explore the efficacy and safety of Actemra for the treatment of patients with COVID-19 pneumonia. However, this new study is vital because there are no well-controlled studies and limited published evidence on the safety or efficacy of Actemra in the treatment of patients suffering from COVID-19.
Currently, there are no FDA-approved treatments for the severe illness caused by SARS-CoV-2. Given the alarming levels of spread and severity, some approved drugs or pipeline candidates are being tested to see if they are effective in treating the infected patients.
Per the recent results published in The New England Journal of Medicine, AbbVie’s (NYSE:ABBV) HIV drug, Kaletra, failed to show any benefit over standard care in adult patients with severe COVID-19 in China. The results came as a disappointment, given the urgent need for treating infected patients.
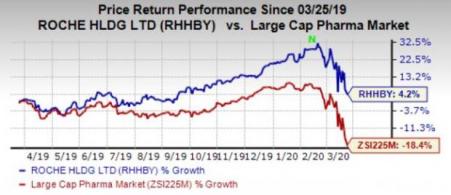
Roche’s stock has gained 4.2% in the past year against the industry’s 18.4% decline.
President Trump reportedly wants to speed up the approval of vaccines and treatments to fight the pandemic.
Regeneron (NASDAQ:REGN) and partner Sanofi (PA:SASY) have also announced a program to evaluate their rheumatoid arthritis drug, Kevzara, to treat patients hospitalized with severe infection due to COVID-19.
Meanwhile, the FDA is also working with government agencies and academic centers, which are evaluating the use of chloroquine to treat patients with mild-to-moderate COVID-19 infection to potentially reduce the duration of symptoms, as well as viral shedding, and help prevent the spread of the disease.
Chloroquine is already approved for treating malaria, lupus and rheumatoid arthritis. Studies are underway to determine the efficacy of using chloroquine to treat COVID-19.
Meanwhile, Gilead Sciences, Inc. (NASDAQ:GILD) has initiated two phase III studies to evaluate the safety and efficacy of remdesivir in adults diagnosed with COVID-19. The candidate was previously under testing for the Ebola virus.
Desperate times call for desperate measures and pharma/biotech companies are running a race against time to successfully develop treatments and vaccines to combat this contagious disease. While the drugs and vaccines will need some time to be tested and a cure is not imminent, investors will keep an eye on these companies as the pandemic is not likely to die out soon.
Roche currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Biggest Tech Breakthrough in a Generation
Be among the early investors in the new type of device that experts say could impact society as much as the discovery of electricity. Current technology will soon be outdated and replaced by these new devices. In the process, it’s expected to create 22 million jobs and generate $12.3 trillion in activity.
A select few stocks could skyrocket the most as rollout accelerates for this new tech. Early investors could see gains similar to buying Microsoft (NASDAQ:MSFT) in the 1990s. Zacks’ just-released special report reveals 8 stocks to watch. The report is only available for a limited time.
See 8 breakthrough stocks now>>
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Gilead Sciences, Inc. (GILD): Free Stock Analysis Report
AbbVie Inc. (ABBV): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Nvidia’s earnings beat didn’t erase investor concerns over slowing growth. Soft Q1 guidance and valuation worries may limit the stock’s upside. Weak network and gaming sales...

Warren Buffett and Berkshire Hathaway (NYSE:BRKa) always make headlines in February when the firm holds its annual meeting. Among the many takeaways is what the company has been...

While Tuesday I wrote about the strength of junk bonds in the face of risk-off ratios (TLT v. SPY, HYG), today, I am still quite concerned about Granny Retail or the consumer...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.