
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Regeneron's SBLA For Eylea Accepted By FDA, Action Date Set

Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN) announced that the FDA has accepted for review the company's supplemental Biologics License Application (sBLA) for the label expansion of Eylea Injection. The company is seeking approval a for a 12-week dosing interval of Eylea (aflibercept) Injection in patients with wet age-related macular degeneration (wet AMD) based on physician's assessment. The action date set by the FDA is Aug 11, 2018.
So far this year, shares of the company have rallied 3.9% compared with the industry’s gain of 3%.
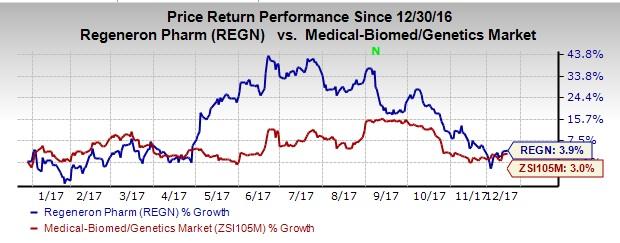
We note that Eylea (aflibercept) is approved in the United States, the EU, Japan and other countries for the treatment of neovascular age-related macular degeneration (wet AMD), diabetic macular edema (DME), macular edema following retinal vein occlusion, which includes macular edema following central retinal vein occlusion and macular edema following branch retinal vein occlusion.
Eylea injection is indicated for the treatment of patients with-neovascular (Wet) age-related macular degeneration (AMD). The recommended dose for Eylea is 2 mg administered by intravitreal injection every 4 weeks (monthly) for the first 12 weeks (3 months), followed by 2 mg once every 8 weeks (2 months).
Eylea may be dosed once per month, but in most patients, additional benefit was not seen with this dosing plan. It is also approved for the treatment of macular edema following Retinal Vein Occlusion (RVO). The recommended dose for Eylea is 2 mg administered by intravitreal injection every 4 weeks (monthly).
It also treats people suffering from diabetic macular edema (DME) and diabetic retinopathy (DR) in patients with DME. The recommended dose is 2 mg administered by intravitreal injection every 4 weeks (monthly) for the first five injections, followed by 2 mg once every 8 weeks (2 months).
The sBLA submission is based on an integrated analysis of two-year results from — VIEW 1 and VIEW 2 — two phase III studies that investigated the treatment of Eylea in patients with wet AMD. The integrated studies found that 51% of patients in the study had their Eylea dosing interval extended to every 12 weeks at the beginning of the second year (week 52) of treatment.
The patients were able to maintain this every 12-week dosing interval and their best-corrected visual acuity (BCVA) gains when they were assessed at the end of the second year (week 96). The second year results confirmed the sustainability of the vision gains achieved by EYLEA with a less than monthly dosing frequency. Thus, if approved for this indication it will mean that the patients will have to take fewer injections of Eylea than before.
Label expansion into additional indications would give Eylea access to higher patient population and increase the commercial potential of the drug.
Meanwhile, Novartis (NYSE:NVS) is developing brolucizumab for treating neovascular (wet) AMD. The candidate in November 2017, demonstrated non-inferiority to Eylea in phase III studies for long-lasting effects in patients. Moreover, it has shown superior improvement in reductions in retinal thickness due to fluid accumulation versus Eylea. A potential approval of brolucizumab will hurt Regeneron’s product sales.
Ophthotech Corp. (NASDAQ:OPHT) is also developing its pipeline candidate, Zimura, in a phase IIa study for treating wet AMD with data expected late next year.
Zacks Rank & Key Pick
Regeneron carries a Zacks Rank #3 (Hold). A better-ranked health care stock in the same space is Johnson & Johnson (NYSE:JNJ) sporting a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Johnson and Johnson’s earnings per share estimates have moved up from $7.19 to $7.28 for 2017 and from $7.75 to $7.85 for 2018, over the last 60 days. The company delivered a positive earnings surprise in all of the trailing four quarters, with an average beat of 3.12%. The share price of the company has increased 22.5% year to date.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple (NASDAQ:AAPL) sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020.
Click here for the 6 trades >>
Novartis AG (NVS): Free Stock Analysis Report
Johnson & Johnson (JNJ): Free Stock Analysis Report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Ophthotech Corporation (OPHT): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Shares of Alibaba (NYSE:BABA) are on a tear to start off 2025. The consumer discretionary and tech stock is up by 52% this year as of the Feb. 25 close. The company’s cloud...

Every investor should know the term CEP, or customer engagement platform, because it is central to businesses' use of AI. CEPs provide software services to connect and communicate...

As markets try to look through the blizzard of policy changes flowing out of Washington, the crowd has shifted its preferences considerably in recent weeks based on a sector lens....
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.