
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Pfizer's Second Biosimilar Of Remicade Receives FDA Approval

Pfizer Inc. (NYSE:PFE) announced that the FDA has approved Ixifi its second biosimilar version of Johnson & Johnson's (NYSE:JNJ) blockbuster rheumatoid arthritis (“RA”) drug, Remicade (infliximab). Notably, Ixifi is a chimeric human-murine monoclonal antibody against tumor necrosis factor.
Ixifi received the nod for all approved indications of Remicade including RA, ulcerative colitis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis and Crohn’s disease.
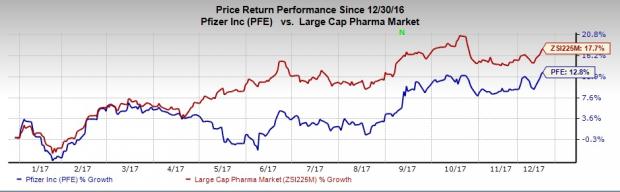
Shares of Pfizer have risen 12.8% so far this year, underperforming the industry’s rally of 17.7%.
The approval was based on an encouraging data from phase III study, REFLECTIONS B537-02. A biologics license application was filed with the FDA in April.
Notably, in February 2016, Pfizer had divested the rights to development, commercialization and manufacturing of Ixifi in the European Economic Area (“EEA”) to Novartis' generic arm, Sandoz. However, Pfizer retains the commercialization and manufacturing rights to Ixifi in all countries outside the EEA.
We remind investors that Pfizer’s first biosimilar product to Remicade, Inflectra, was launched last November in the United States. The company has exclusive commercialization rights to Inflectra in the United States and on certain other territories.
In September, Pfizer filed a lawsuit in a U.S. district court alleging that J&J is resorting to unfair practices to prevent the sale of Inflectra, available at a Wholesale Acquisition Cost, which is 19% lower than J&J’s referenced product.
Pfizer alleged in a complaint filed in the U.S. District Court for the Eastern District of Pennsylvania that J&J has entered into biosimilar-exclusion contracts with U.S. insurers. These contracts boost insurers not to cover Inflectra, thus making it difficult to access by patients and protecting its referenced product.
Pfizer also claimed that anticompetitive contracts with discounts were offered to hospitals on purchase of Remicade on condition that they do not buy the biosimilar versions. Pfizer claims that these unfair practices benefit sales of J&J’s referenced product, a key revenue generator for the latter.
Pfizer is evaluating 13 biosimilar molecules in various stages of development. Biosimilar products in late-stage development include biosimilar versions of Roche's (OTC:RHHBY) Rituxan and Avastin and AbbVie's (NYSE:ABBV) Humira. A biosimilar version of Herceptin is under review in the United States and the EU. In Europe, Pfizer markets Nivestim, biosimilar versions of Amgen’s Neupogen and Retacrit as well as biosimilar versions of Amgen’s Epogen.
Zacks Rank
Pfizer carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Zacks Editor-in-Chief Goes "All In" on This Stock
Full disclosure, Kevin Matras now has more of his own money in one particular stock than in any other. He believes in its short-term profit potential and also in its prospects to more than double by 2019. Today he reveals and explains his surprising move in a new Special Report.
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Pfizer, Inc. (PFE): Free Stock Analysis Report
Johnson & Johnson (JNJ): Free Stock Analysis Report
AbbVie Inc. (ABBV): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Warren Buffett and Berkshire Hathaway (NYSE:BRKa) always make headlines in February when the firm holds its annual meeting. Among the many takeaways is what the company has been...

While Tuesday I wrote about the strength of junk bonds in the face of risk-off ratios (TLT v. SPY, HYG), today, I am still quite concerned about Granny Retail or the consumer...

Shares of Caesars Entertainment (NASDAQ:CZR), a leading gambling stock, traded around 3% higher on Wednesday morning, though the stock was trading around 1.5% lower shortly before...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.