
- All Instrument Types
- Indices
- Equities
- ETFs
- Funds
- Commodities
- Currencies
- Crypto
- Bonds
- Certificates
Please try another search

Pfizer's Arthritis Drug Gets FDA Nod For Label Expansion

Pfizer Inc. (NYSE:PFE) announced that the FDA has approved a label expansion for its janus kinase (JAK) inhibitor, Xeljanz (tofacitinib), for two doses — 5 mg twice daily and 11 mg once-daily extended-release (XR) formulation.
With this latest approval, Xeljanz can now be administered for treating adult patients with active psoriatic arthritis (PsA), who had an inadequate response to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and tumor necrosis factor inhibitors, respectively.
Notably, Xeljanz (5 mg twice daily) is approved in the United States as a second-line treatment option for patients with moderate-to-severely active rheumatoid arthritis (“RA”), who have had an inadequate response to or cannot tolerate methotrexate (MTX). The drug was approved in Europe and China in March for RA. Significantly, this is the first and only JAK inhibitor approved in the United States for both moderate to severe RA and an active PsA.
Shares of Pfizer have gained 12.3% so far this year, underperforming the industry’s rally of 16.5%.
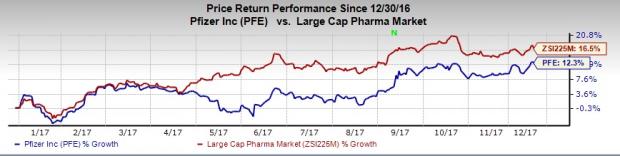
A supplemental new drug application (sNDA) for Xeljanz was filed with the FDA in May this year for the aforementioned indication. The approval was based on encouraging data from two phase III OPAL (Oral Psoriatic Arthritis TriaL) studies — Broaden and Beyond — on Xeljanz.
Both OPAL Broaden and OPAL Beyond trials evaluated the efficacy and safety of Xeljanz on adult patients with PsA. The programs achieved the primary efficacy endpoint of a statistically significant improvement with twice-daily Xeljanz 5 mg compared with placebo at three months as per measurement by American College of Rheumatology 20 response and the change from baseline in the Health Assessment Questionnaire Disability Index score.
Overall safety results from these studies were found to be consistent with those observed in the broader rheumatology development program for Xeljanz.
We remind investors that the company is also looking to get the drug’s label expanded to include treatment of adult patients suffering from moderate-to-severe active ulcerative colitis. A response from the FDA is expected in March next year.
Additionally, Pfizer is conducting a phase IIIb/IV head-to-head study comparing Xeljanz with AbbVie's (NYSE:ABBV) Humira. In February, the company announced top-line results from the assessment. Outcomes from the trial demonstrated non-inferiority of combo therapy comprising Xeljanz plus MTX compared with the other combo agents Humira plus MTX, thus meeting the primary endpoint.
We are encouraged by Pfizer’s label expansion efforts. Xeljanz’s U.S. sales surged 44% year over year to $935 million in the first nine months of 2017. The drug’s label expansion should boost further the sales.
Blockbuster drugs approved to treat PsA and RA include Johnson & Johnson's (NYSE:JNJ) Remicade and Amgen, Inc.'s (NASDAQ:AMGN) Enbrel that Pfizer markets outside the United States and Canada.
In another Pfizer press release, the company announced that it has started a phase III study, evaluating the efficacy and safety of its once-daily janus kinase 1 (JAK1) inhibitor, PF-04965842, for treatment of moderate-to-severe atopic dermatitis. This initiation surfaces as the first trial in the JAK1 Atopic Dermatitis Efficacy and safety global development program.
Zacks Rank
Pfizer carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Investor Alert: Breakthroughs Pending
A medical advance is now at the flashpoint between theory and realization. Billions of dollars in research have poured into it. Companies are already generating substantial revenue, and even more wondrous products are in the pipeline.
Cures for a variety of deadly diseases are in sight, and so are big potential profits for early investors. Zacks names 5 stocks to buy now.
Pfizer, Inc. (PFE): Free Stock Analysis Report
Johnson & Johnson (JNJ): Free Stock Analysis Report
AbbVie Inc. (ABBV): Free Stock Analysis Report
Amgen Inc. (AMGN): Free Stock Analysis Report
Original post
Zacks Investment Research
Related Articles

Shares of Caesars Entertainment (NASDAQ:CZR), a leading gambling stock, traded around 3% higher on Wednesday morning, though the stock was trading around 1.5% lower shortly before...

Amazon (NASDAQ:AMZN) is making a significant push into the future with a robust investment in robotics and artificial intelligence. The company has earmarked $35 billion for...

Home Depot’s (NYSE:HD) Q4 2024 report and guidance for 2025 have plenty to be unhappy about, but the simple truth is that this company turned a corner in 2024. It is on track for...
Are you sure you want to block %USER_NAME%?
By doing so, you and %USER_NAME% will not be able to see any of each other's Investing.com's posts.
%USER_NAME% was successfully added to your Block List
Since you’ve just unblocked this person, you must wait 48 hours before renewing the block.
I feel that this comment is:
Thank You!
Your report has been sent to our moderators for review





Add a Comment
We encourage you to use comments to engage with other users, share your perspective and ask questions of authors and each other. However, in order to maintain the high level of discourse we’ve all come to value and expect, please keep the following criteria in mind:
Enrich the conversation, don’t trash it.
Stay focused and on track. Only post material that’s relevant to the topic being discussed.
Be respectful. Even negative opinions can be framed positively and diplomatically. Avoid profanity, slander or personal attacks directed at an author or another user. Racism, sexism and other forms of discrimination will not be tolerated.
Perpetrators of spam or abuse will be deleted from the site and prohibited from future registration at Investing.com’s discretion.